Deposition Date
2001-07-17
Release Date
2002-07-11
Last Version Date
2023-12-13
Entry Detail
PDB ID:
1EAZ
Keywords:
Title:
Crystal structure of the phosphoinositol (3,4)-bisphosphate binding PH domain of TAPP1 from human.
Biological Source:
Source Organism:
HOMO SAPIENS (Taxon ID: 9606)
Host Organism:
Method Details:
Experimental Method:
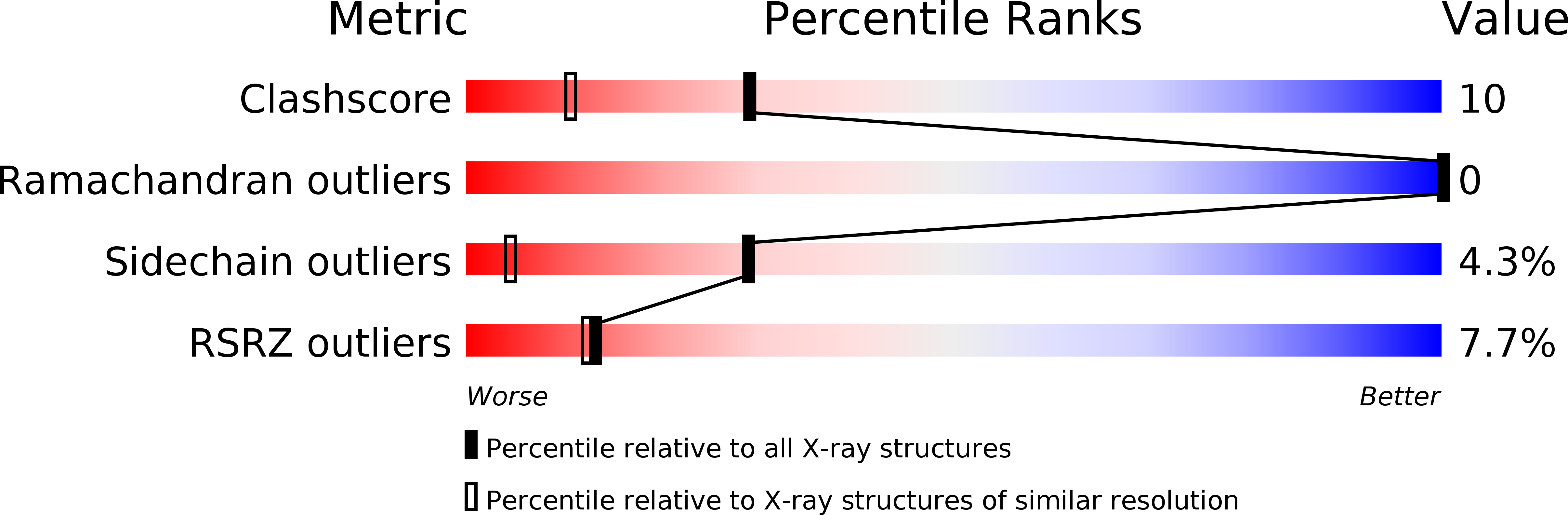
Resolution:
1.40 Å
R-Value Free:
0.22
R-Value Observed:
0.17
Space Group:
C 2 2 21