Deposition Date
1994-11-22
Release Date
1995-02-07
Last Version Date
2024-10-30
Entry Detail
PDB ID:
1EAU
Keywords:
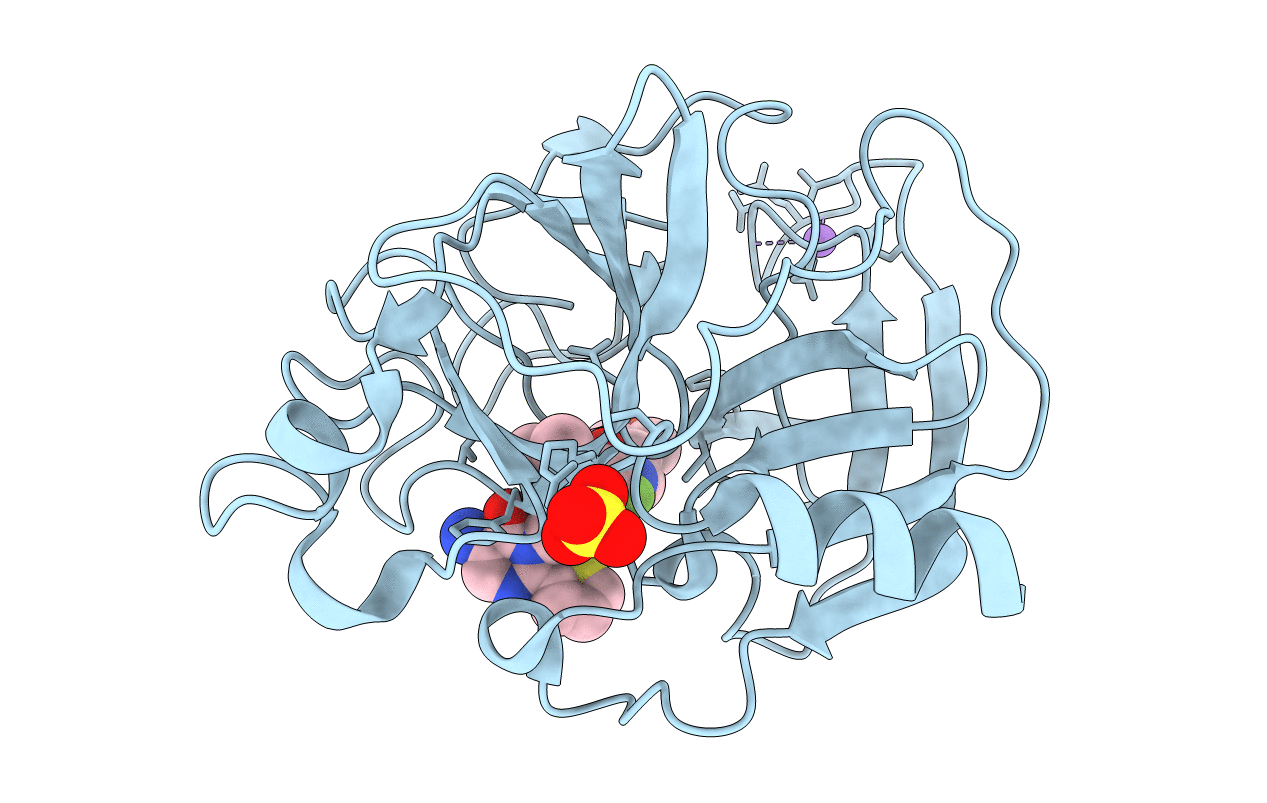
Title:
NONPEPTIDIC INHIBITORS OF HUMAN LEUKOCYTE ELASTASE. 6. DESIGN OF A POTENT, INTRATRACHEALLY ACTIVE, PYRIDONE-BASED TRIFLUOROMETHYL KETONE
Biological Source:
Source Organism:
Sus scrofa (Taxon ID: 9823)
Method Details:
Experimental Method:
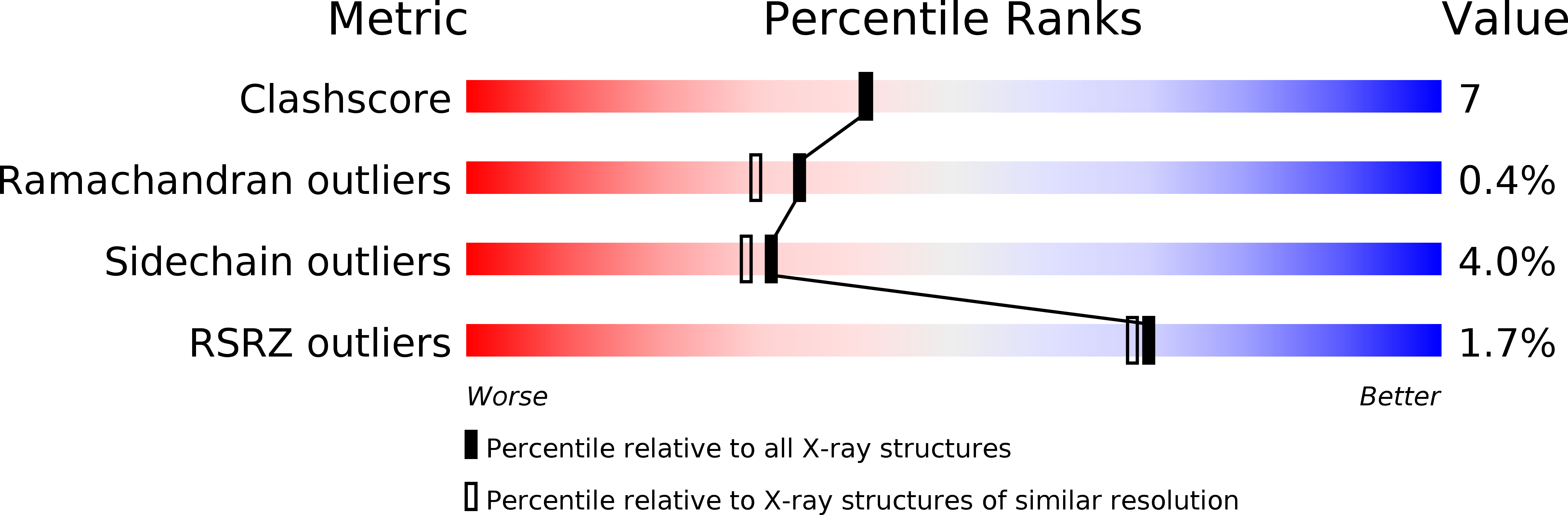
Resolution:
2.00 Å
R-Value Work:
0.16
R-Value Observed:
0.16
Space Group:
P 21 21 21