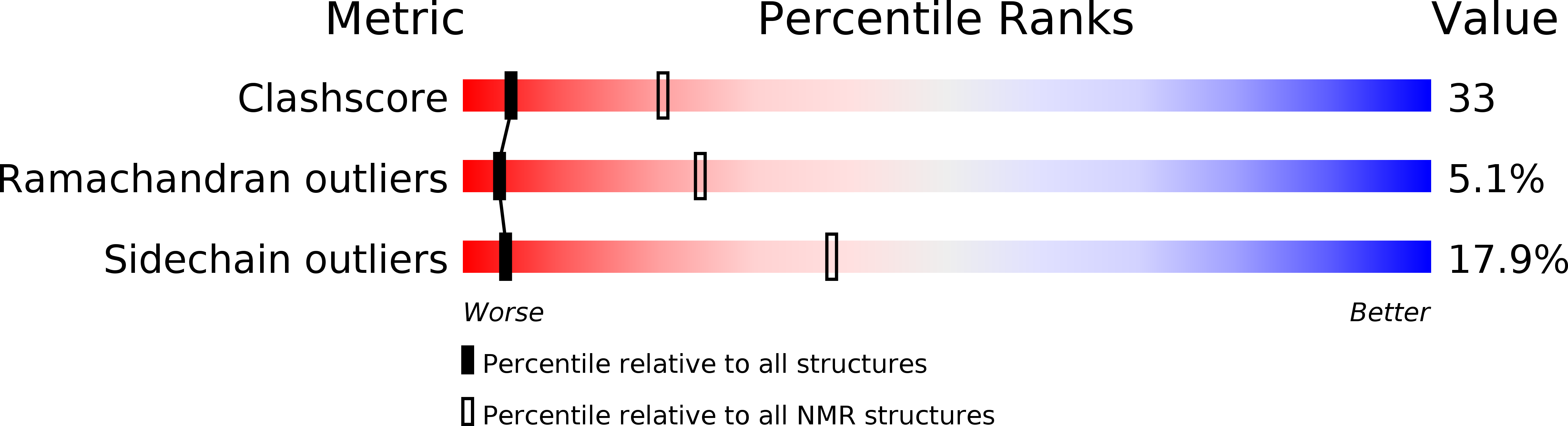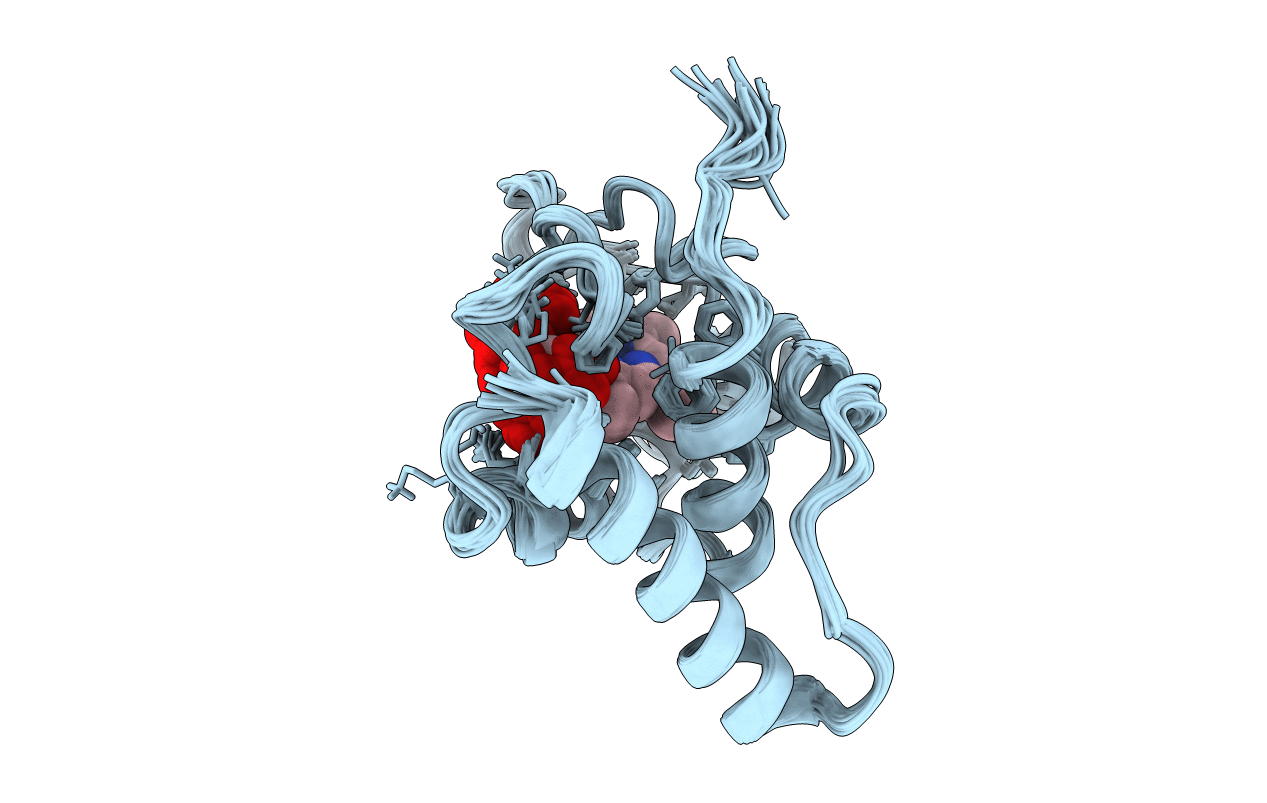
Deposition Date
2000-09-20
Release Date
2001-09-20
Last Version Date
2024-10-23
Entry Detail
PDB ID:
1E8E
Keywords:
Title:
Solution Structure of Methylophilus methylotrophus Cytochrome c''. Insights into the Structural Basis of Haem-Ligand Detachment
Biological Source:
Source Organism:
METHYLOPHILUS METHYLOTROPHUS (Taxon ID: 17)
Method Details:
Experimental Method:
Conformers Calculated:
400
Conformers Submitted:
20
Selection Criteria:
LEAST RESTRAINT VIOLATION