Deposition Date
2000-07-26
Release Date
2001-07-26
Last Version Date
2023-12-13
Entry Detail
PDB ID:
1E5H
Keywords:
Title:
DELTA-R307A DEACETOXYCEPHALOSPORIN C SYNTHASE COMPLEXED WITH SUCCINATE AND CARBON DIOXIDE
Biological Source:
Source Organism:
STREPTOMYCES CLAVULIGERUS (Taxon ID: 1901)
Host Organism:
Method Details:
Experimental Method:
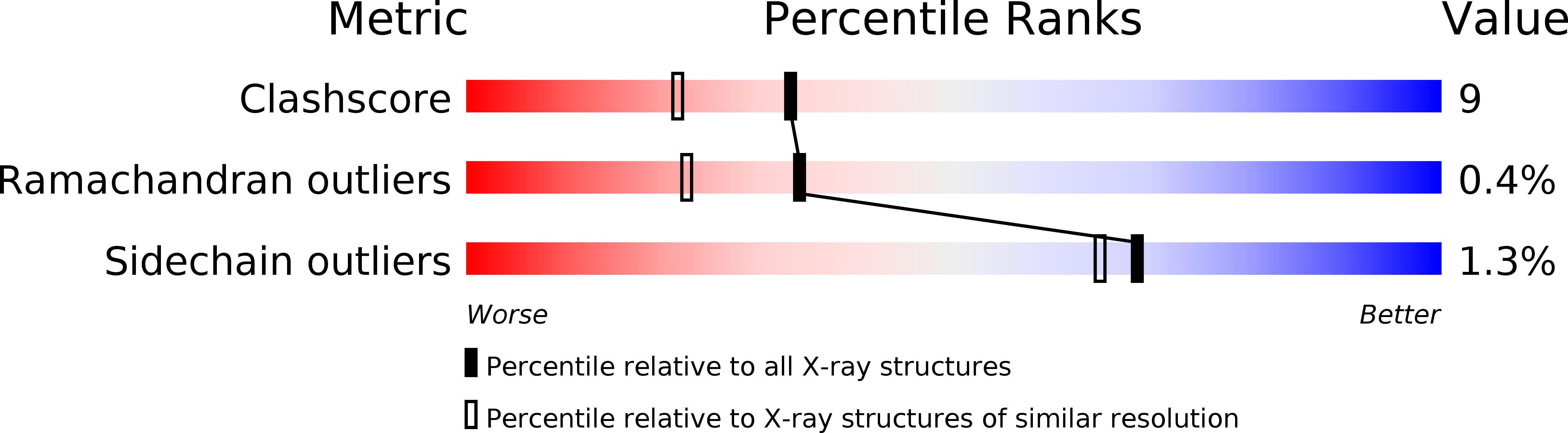
Resolution:
1.96 Å
R-Value Free:
0.25
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
H 3