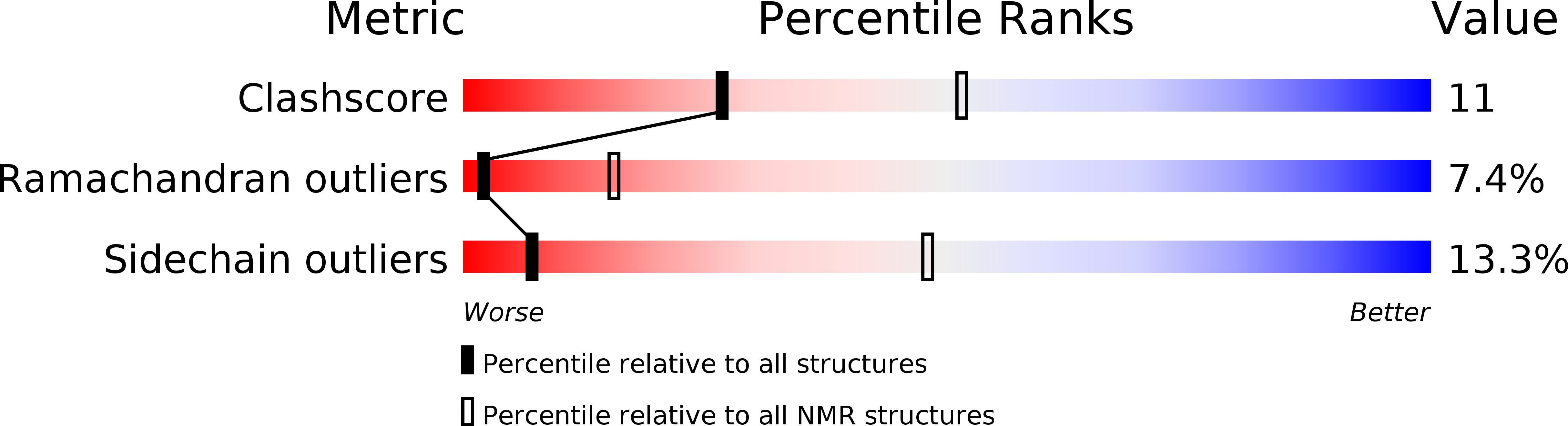Deposition Date
2000-02-24
Release Date
2000-03-16
Last Version Date
2024-05-15
Entry Detail
PDB ID:
1DZD
Keywords:
Title:
High resolution structure of acidic fibroblast growth factor (27-154), 24 NMR structures
Biological Source:
Source Organism(s):
HOMO SAPIENS (Taxon ID: 9606)
Expression System(s):
Method Details: