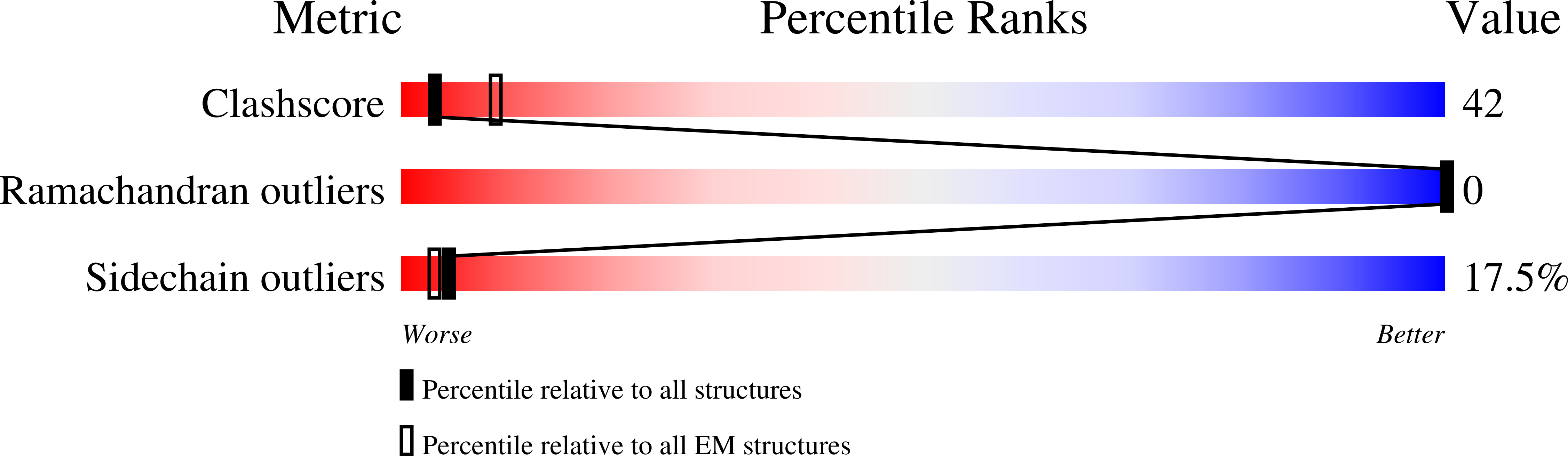
Deposition Date
2000-02-02
Release Date
2000-08-18
Last Version Date
2024-10-16
Entry Detail
PDB ID:
1DYL
Keywords:
Title:
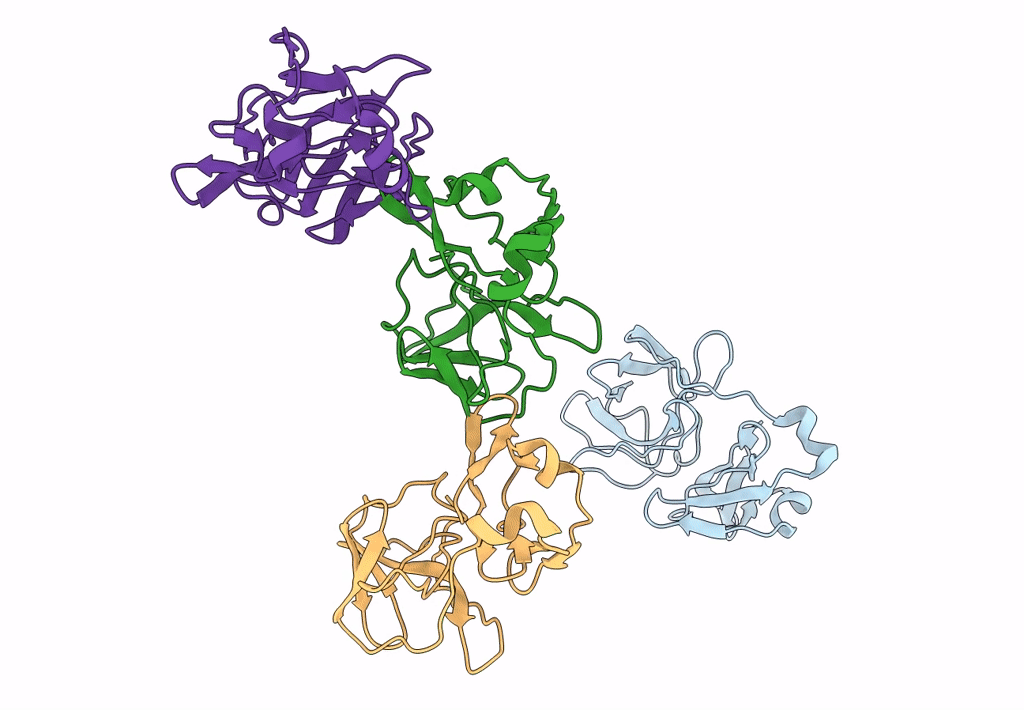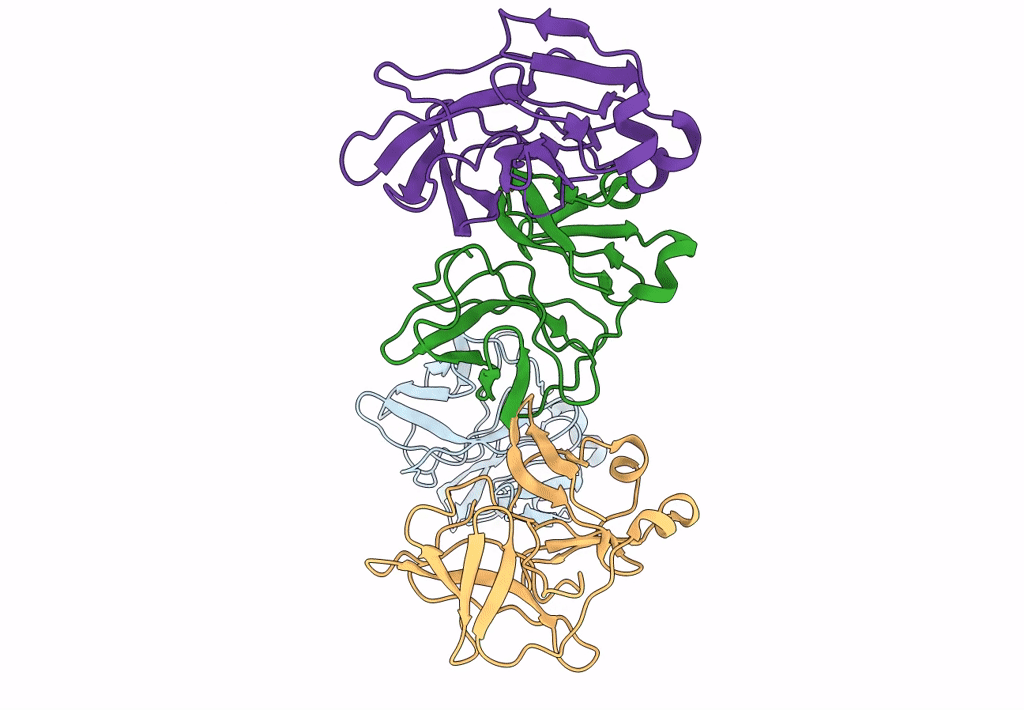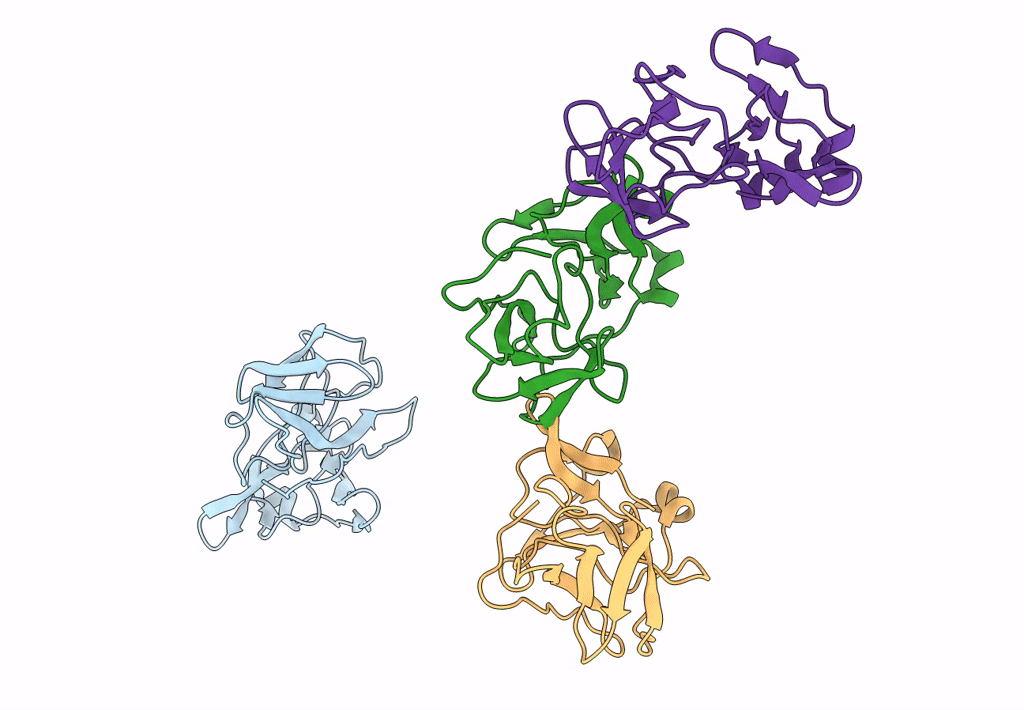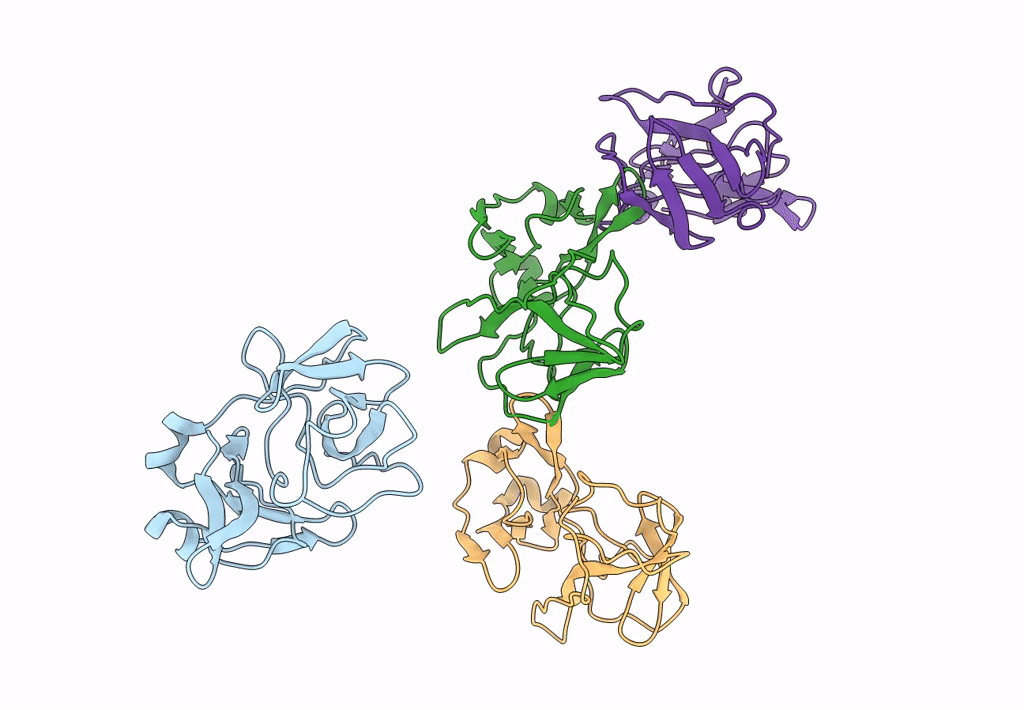
9 ANGSTROM RESOLUTION CRYO-EM RECONSTRUCTION STRUCTURE OF SEMLIKI FOREST VIRUS (SFV) AND FITTING OF THE CAPSID PROTEIN STRUCTURE IN THE EM DENSITY
Biological Source:
Source Organism(s):
SEMLIKI FOREST VIRUS (Taxon ID: 11033)
Method Details:
Experimental Method:
Resolution:
9.00 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE