Abstact
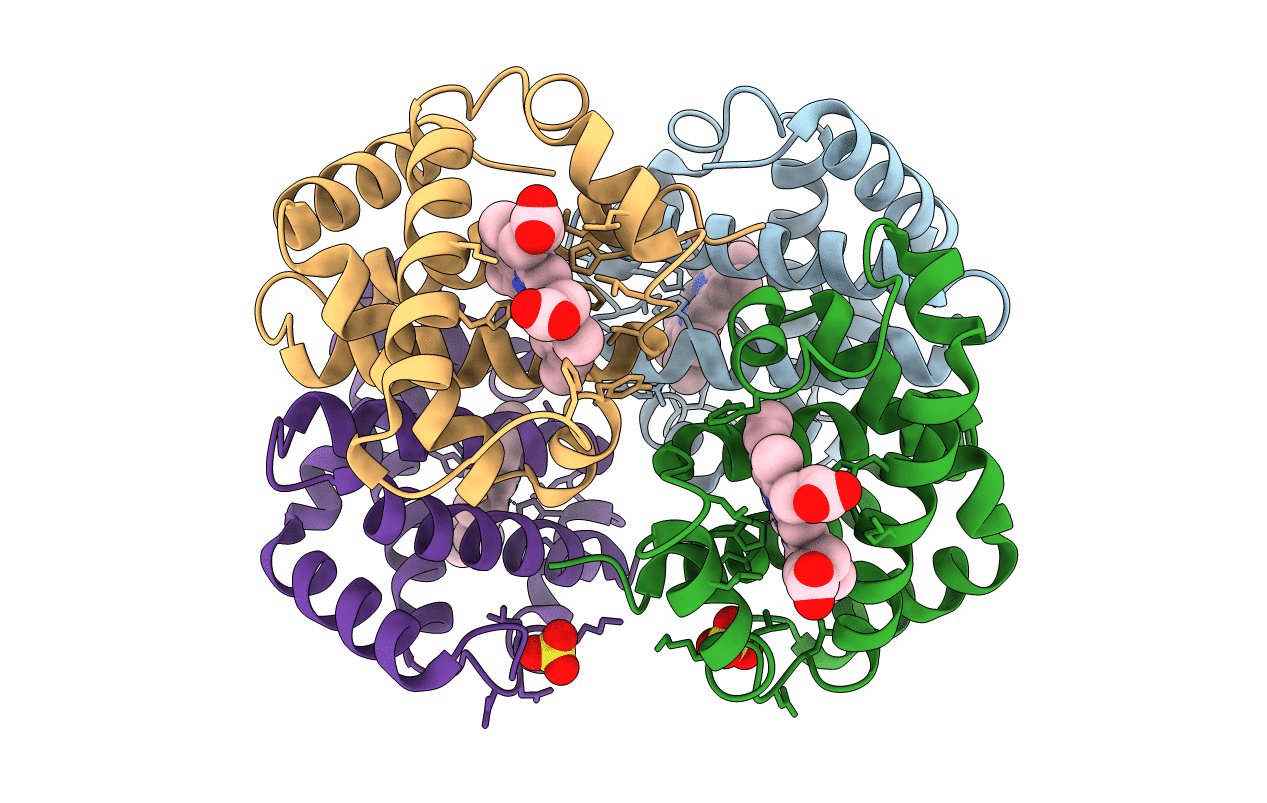
The crystal structures of three mutant hemoglobins reconstituted from recombinant beta chains and authentic human alpha chains have been determined in the deoxy state at 1.8-A resolution. The primary structures of the mutant hemoglobins differ at the beta-chain amino terminus. One mutant, beta Met, is characterized by the addition of a methionine at the amino terminus. The other two hemoglobins are characterized by substitution of Val 1 beta with either a methionine, beta V1M, or an alanine, beta V1A. All the mutation-induced structural perturbations are small intrasubunit changes that are localized to the immediate vicinity of the beta-chain amino terminus. In the beta Met and beta V1A mutants, the mobility of the beta-chain amino terminus increases and the electron density of an associated inorganic anion is decreased. In contrast, the beta-chain amino terminus of the beta V1M mutant becomes less mobile, and the inorganic anion binds with increased affinity. These structural differences can be correlated with functional data for the mutant hemoglobins [Doyle, M. L., Lew, G., DeYoung, A., Kwiatkowski, L., Noble, R. W., & Ackers, G. K. (1992) Biochemistry preceding paper is this issue] as well as with the properties of ruminant hemoglobins and a mechanism [Perutz, M., & Imai, K. (1980) J. Mol. Biol. 136, 183-191] that relates the intrasubunit interactions of the beta-chain amino terminus to changes in oxygen affinity. Since the structures of the mutant deoxyhemoglobins show only subtle differences from the structure of deoxyhemoglobin A, it is concluded that any of the three hemoglobins could probably function as a surrogate for hemoglobin A.(ABSTRACT TRUNCATED AT 250 WORDS)