Deposition Date
2000-01-13
Release Date
2001-01-12
Last Version Date
2023-12-06
Entry Detail
PDB ID:
1DXP
Keywords:
Title:
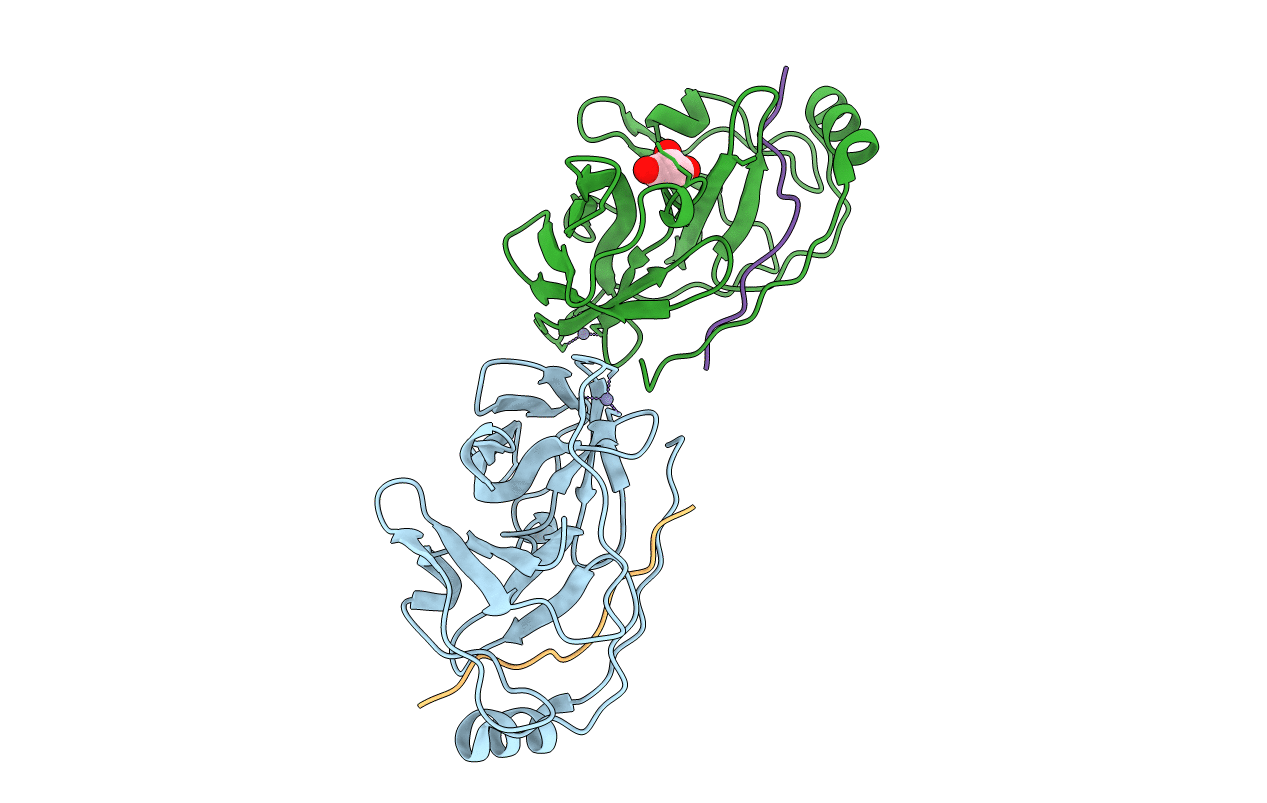
Inhibition of the Hepatitis C Virus NS3/4A Protease. The Crystal Structures of Two Protease-Inhibitor Complexes (apo structure)
Biological Source:
Source Organism(s):
HEPATITIS C VIRUS (ISOLATE TAIWAN) (Taxon ID: 31645)
Expression System(s):
Method Details:
Experimental Method:
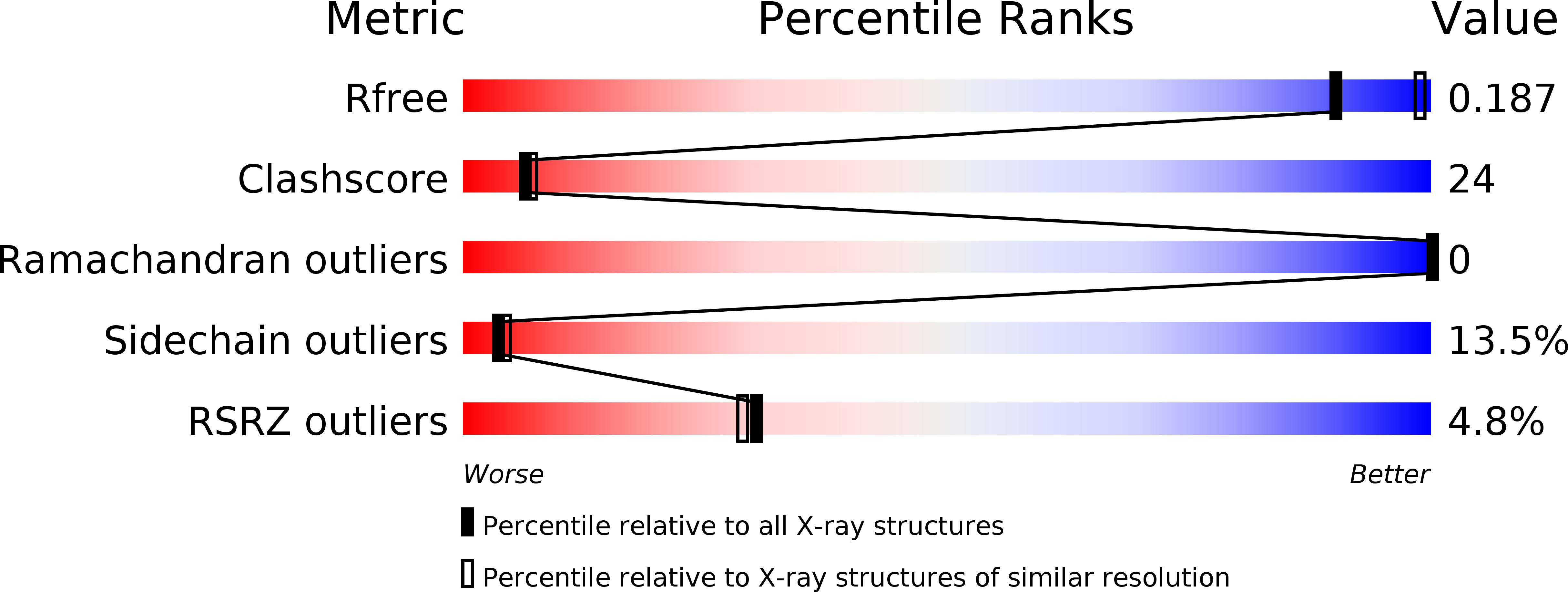
Resolution:
2.40 Å
R-Value Free:
0.29
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 61