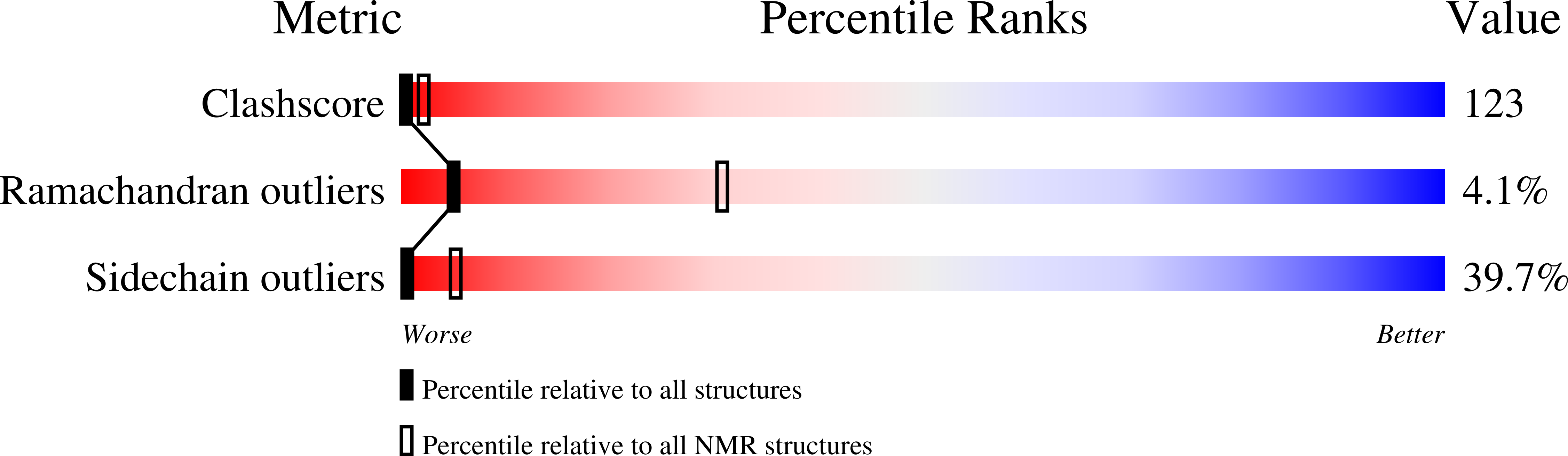
Deposition Date
2000-01-18
Release Date
2001-06-27
Last Version Date
2024-05-22
Entry Detail
PDB ID:
1DUM
Keywords:
Title:
NMR STRUCTURE OF [F5Y, F16W] MAGAININ 2 BOUND TO PHOSPHOLIPID VESICLES
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
10
Selection Criteria:
structures with the lowest energy