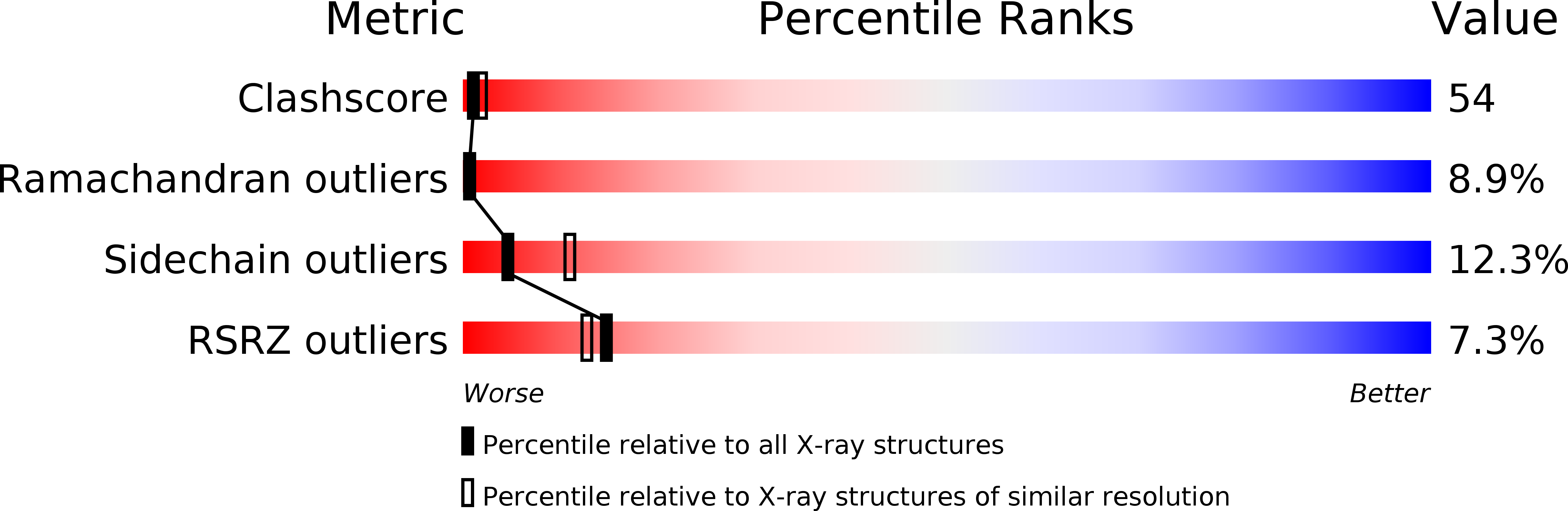
Deposition Date
2000-01-12
Release Date
2000-02-02
Last Version Date
2024-02-07
Entry Detail
PDB ID:
1DT9
Keywords:
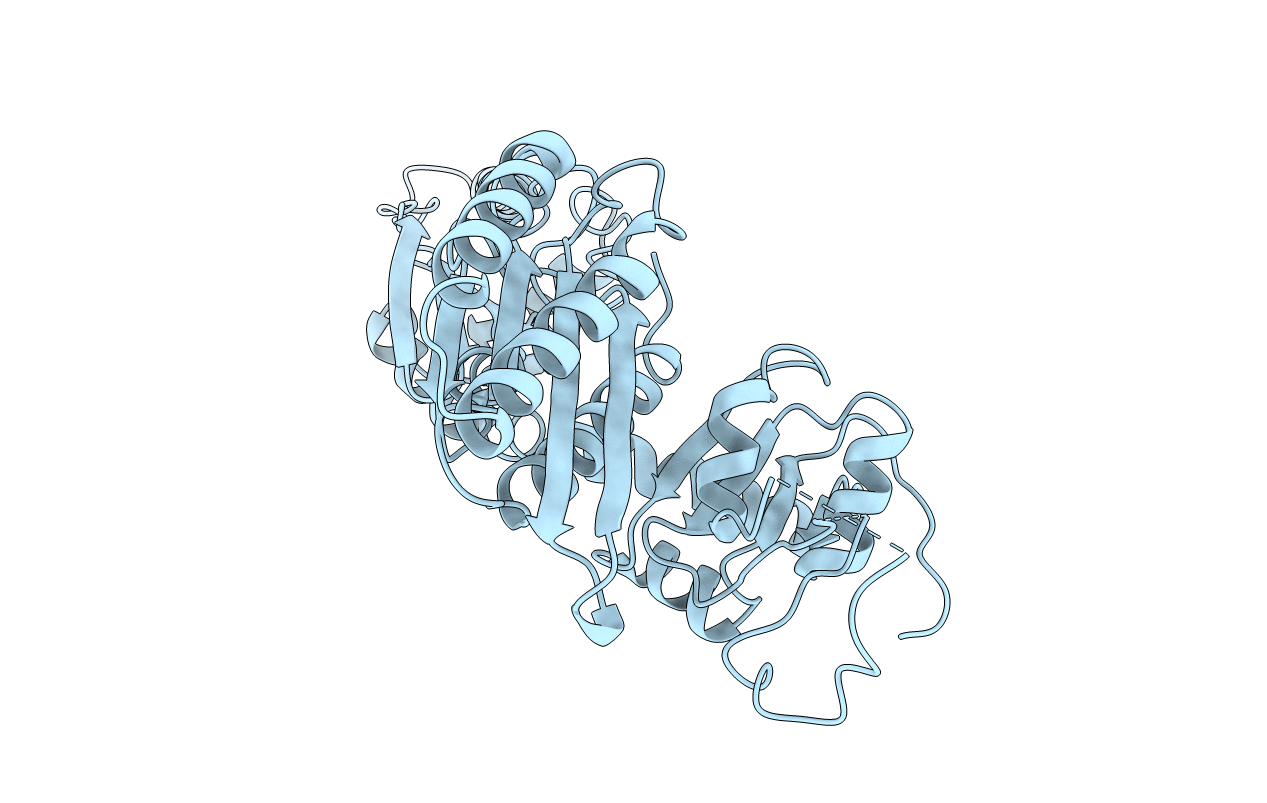
Title:
THE CRYSTAL STRUCTURE OF HUMAN EUKARYOTIC RELEASE FACTOR ERF1-MECHANISM OF STOP CODON RECOGNITION AND PEPTIDYL-TRNA HYDROLYSIS
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Method Details:
Experimental Method:
Resolution:
2.70 Å
R-Value Free:
0.31
R-Value Work:
0.24
Space Group:
P 43 21 2