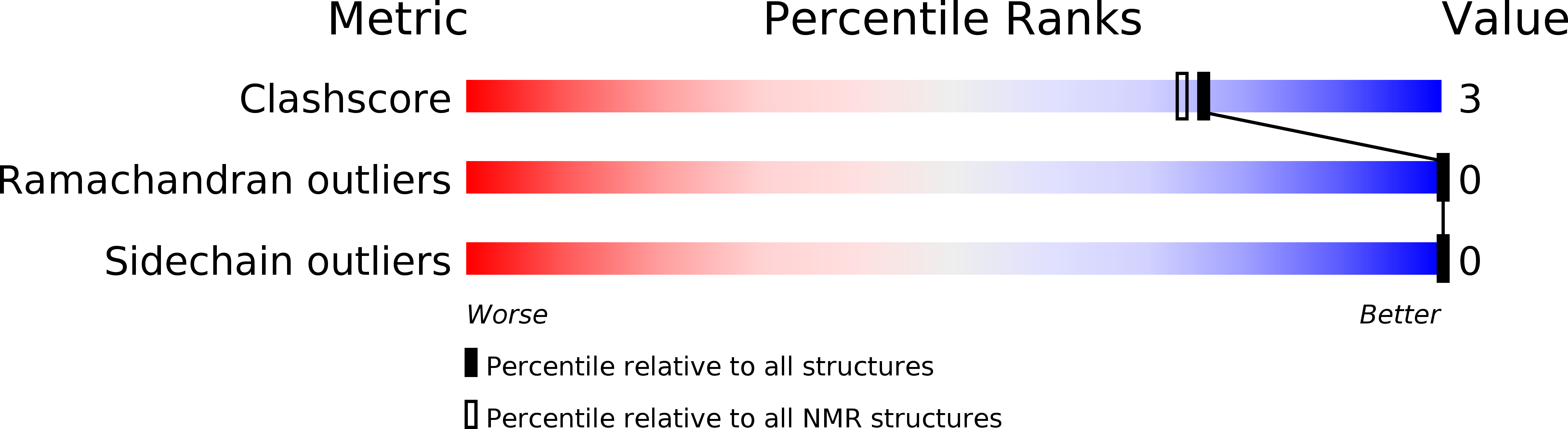
Deposition Date
1996-07-05
Release Date
1997-02-12
Last Version Date
2023-11-15
Method Details:
Experimental Method:
Conformers Calculated:
450
Conformers Submitted:
6
Selection Criteria:
ENERGY MINIMIZED SNAPSHOT EVERY 75 PS