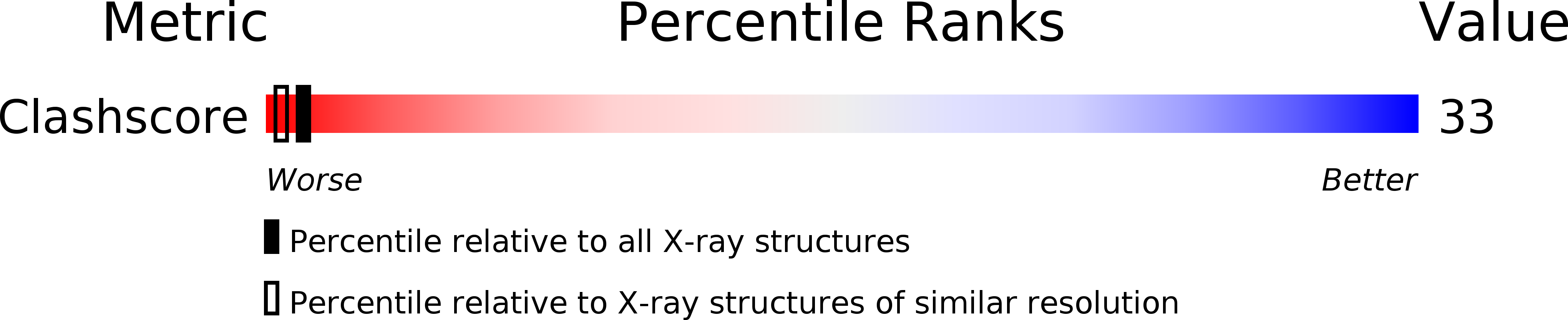Deposition Date
1989-02-22
Release Date
1990-04-15
Last Version Date
2024-02-07
Entry Detail
PDB ID:
1DNS
Keywords:
Title:
BASE ONLY BINDING OF SPERMINE IN THE DEEP GROOVE OF THE A-DNA OCTAMER D(GTGTACAC)
Method Details:
Experimental Method:
Resolution:
2.00 Å
R-Value Observed:
0.11
Space Group:
P 43 21 2