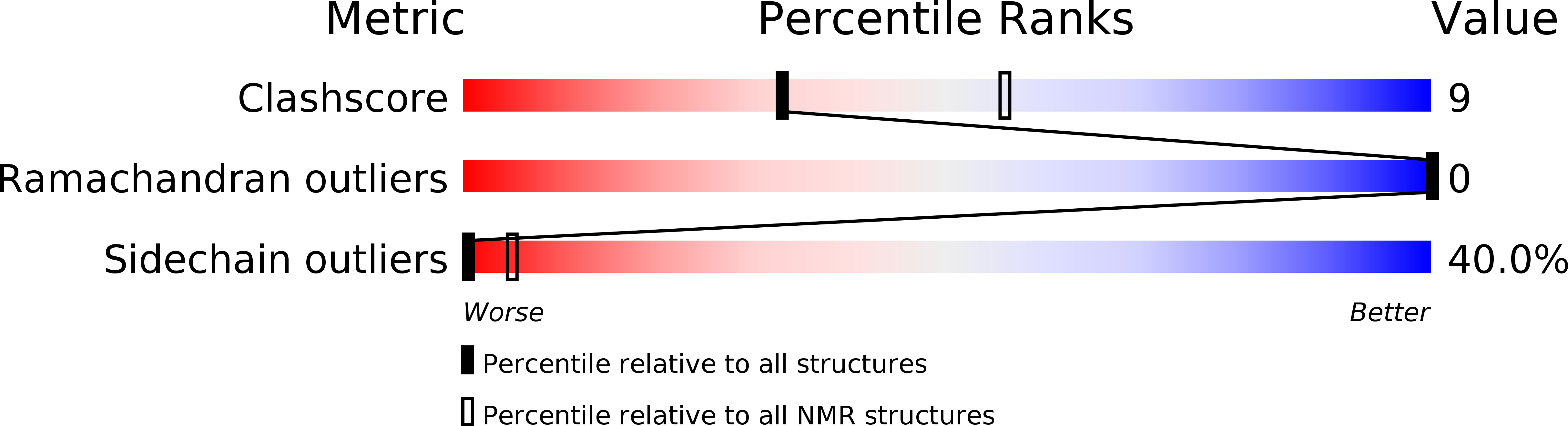
Deposition Date
1999-12-16
Release Date
2000-01-12
Last Version Date
2024-04-10
Entry Detail
PDB ID:
1DNG
Keywords:
Title:
NMR STRUCTURE OF A MODEL HYDROPHILIC AMPHIPATHIC HELICAL ACIDIC PEPTIDE
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Method Details:
Experimental Method:
Conformers Calculated:
50
Conformers Submitted:
1
Selection Criteria:
BACK CALCULATED DATA AGREE WITH EXPERIMENTAL NOESY SPECTRUM,STRUCTURES WITH THE LEAST RESTRAINT VIOLATIONS,STRUCTURES WITH THE LOWEST ENERGY