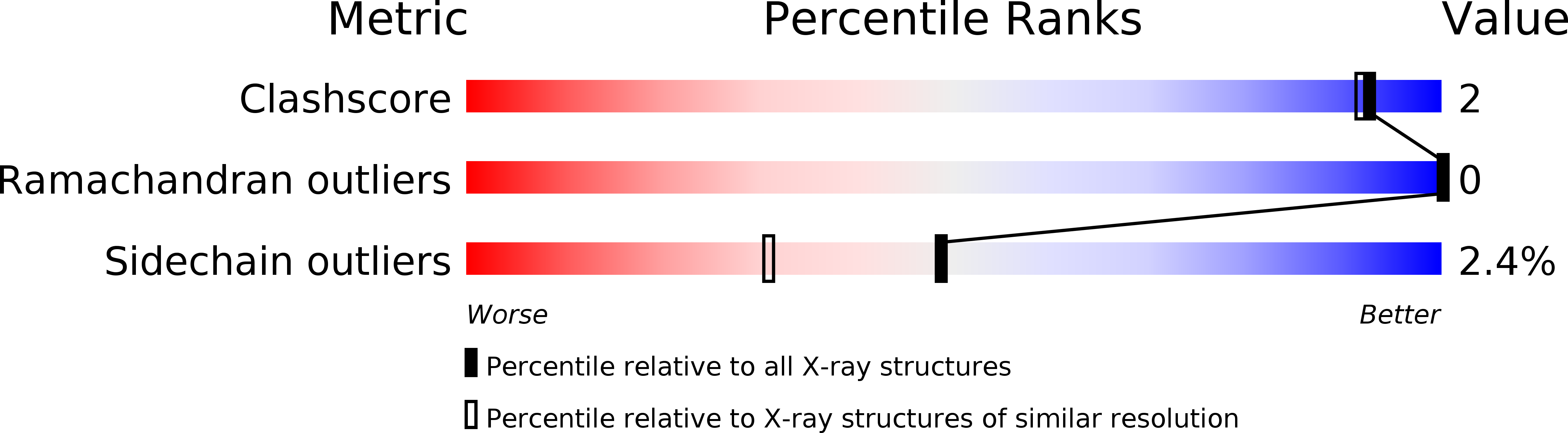
Deposition Date
1995-10-09
Release Date
1996-03-08
Last Version Date
2024-06-05
Entry Detail
PDB ID:
1DIF
Keywords:
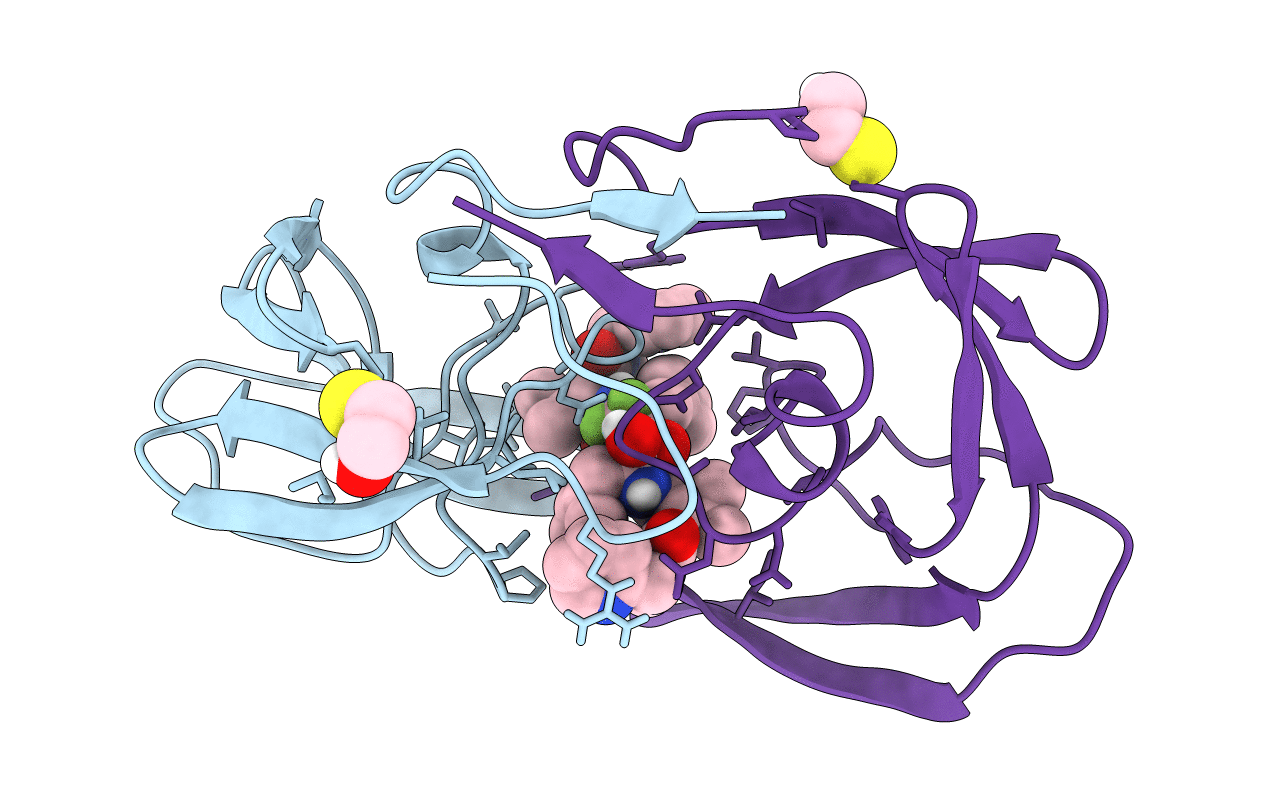
Title:
HIV-1 PROTEASE IN COMPLEX WITH A DIFLUOROKETONE CONTAINING INHIBITOR A79285
Biological Source:
Source Organism(s):
Human immunodeficiency virus 1 (Taxon ID: 11676)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
1.70 Å
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 21 21 21