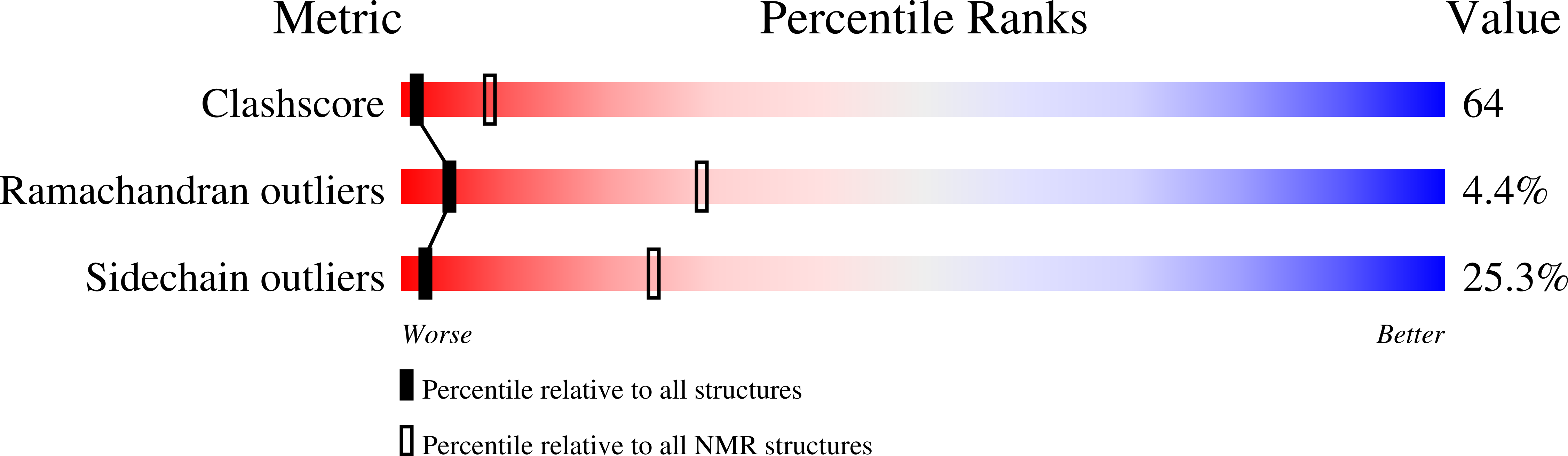
Deposition Date
1999-10-28
Release Date
2000-10-28
Last Version Date
2024-05-22
Entry Detail
PDB ID:
1D9N
Keywords:
Title:
SOLUTION STRUCTURE OF THE METHYL-CPG-BINDING DOMAIN OF THE METHYLATION-DEPENDENT TRANSCRIPTIONAL REPRESSOR MBD1/PCM1
Biological Source:
Source Organism:
Homo sapiens (Taxon ID: 9606)
Host Organism:
Method Details:
Experimental Method:
Conformers Calculated:
125
Conformers Submitted:
25
Selection Criteria:
structures with the lowest energy