Deposition Date
1999-10-27
Release Date
1999-12-02
Last Version Date
2024-02-07
Entry Detail
PDB ID:
1D9F
Keywords:
Title:
CRYSTAL STRUCTURE OF THE COMPLEX OF DNA POLYMERASE I KLENOW FRAGMENT WITH DNA TETRAMER CARRYING 2'-O-(3-AMINOPROPYL)-RNA MODIFICATION 5'-D(TT)-AP(U)-D(T)-3'
Biological Source:
Source Organism(s):
Escherichia coli (Taxon ID: 562)
Expression System(s):
Method Details:
Experimental Method:
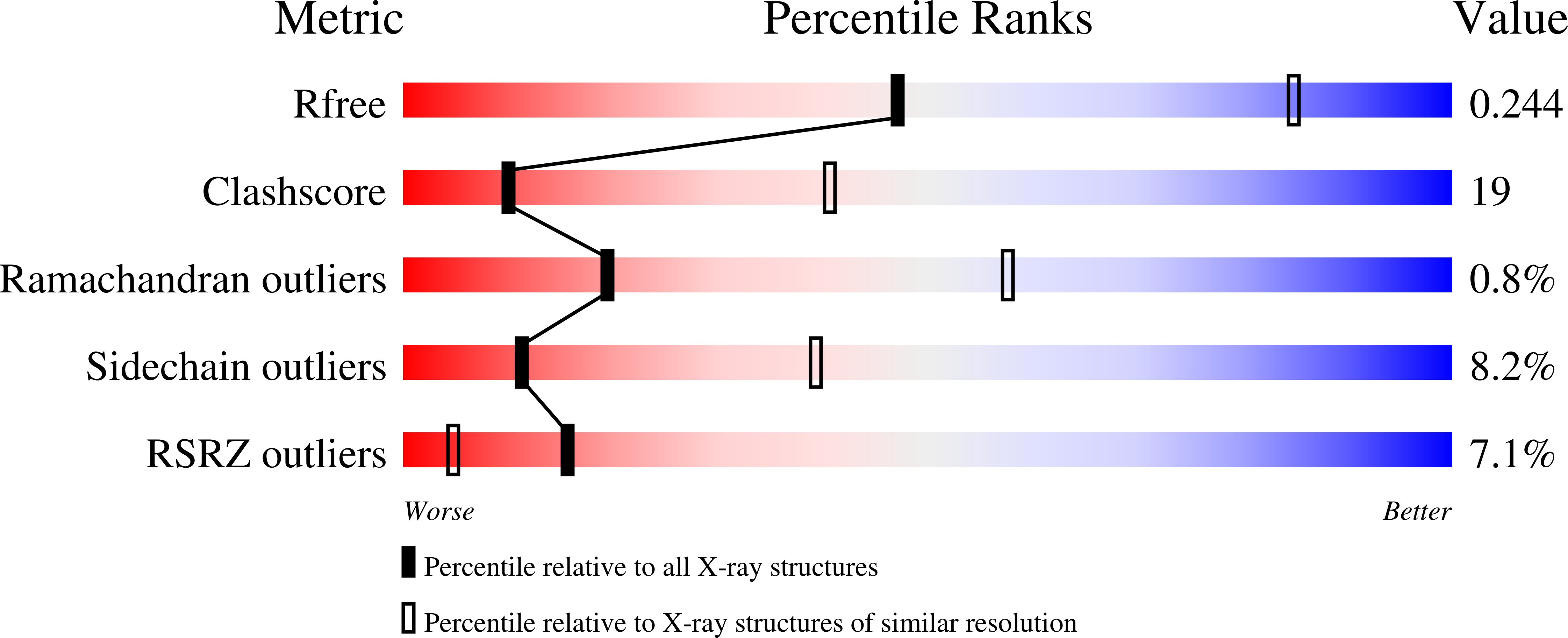
Resolution:
3.00 Å
R-Value Free:
0.24
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
P 43