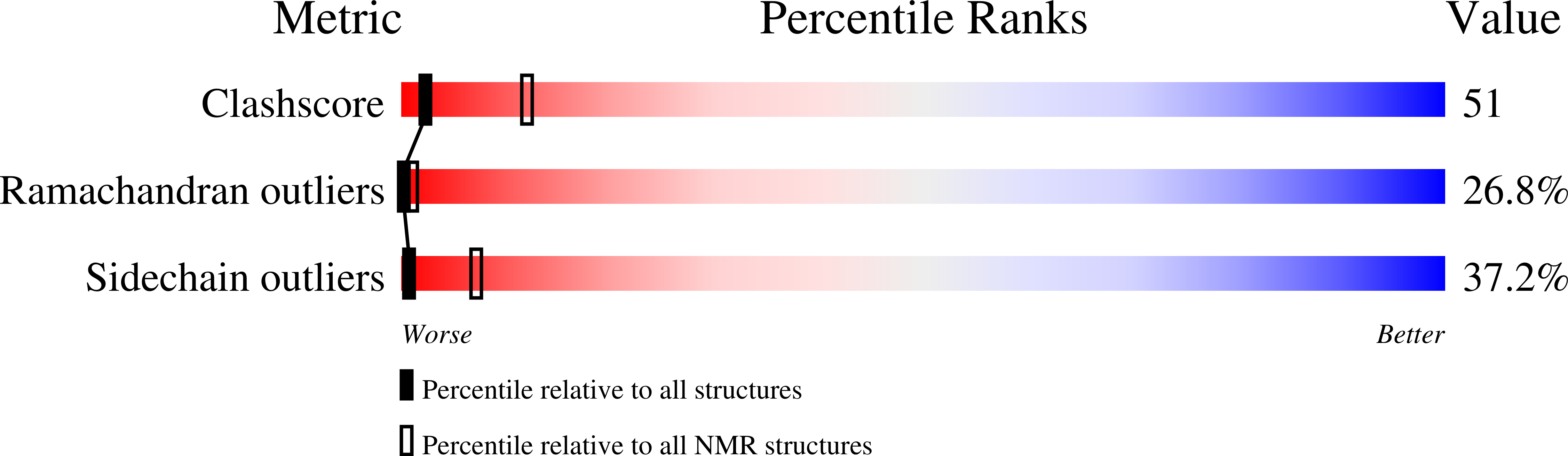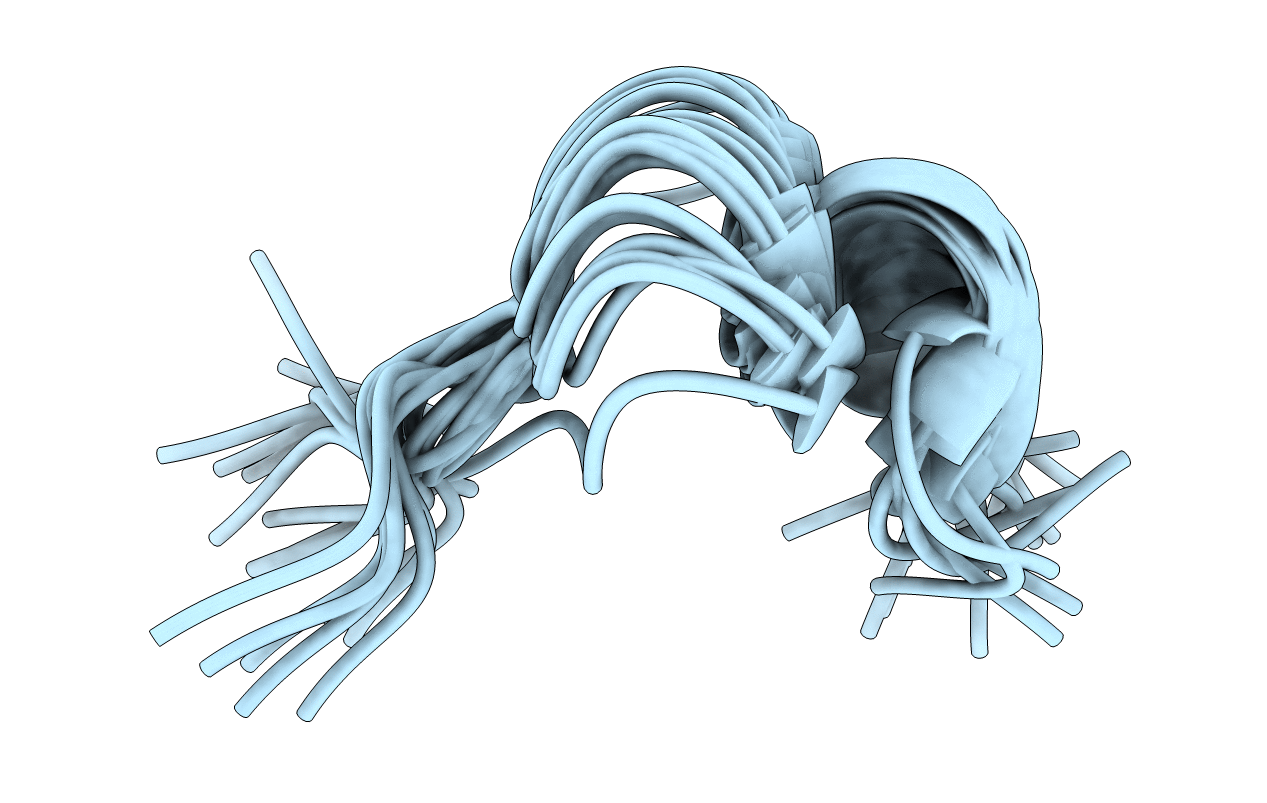
Deposition Date
1999-10-15
Release Date
2000-01-03
Last Version Date
2024-05-22
Entry Detail
PDB ID:
1D6X
Keywords:
Title:
THE STRUCTURE OF THE ANTIMICROBIAL PEPTIDE TRITRPTICIN BOUND TO MICELLES-A DISTINCT MEMBRANE-BOUND PEPTIDE FOLD
Method Details:
Experimental Method:
Conformers Calculated:
97
Conformers Submitted:
19
Selection Criteria:
structures with favorable non-bond energy