Deposition Date
1999-10-11
Release Date
2000-10-11
Last Version Date
2024-11-20
Entry Detail
PDB ID:
1D5Q
Keywords:
Title:
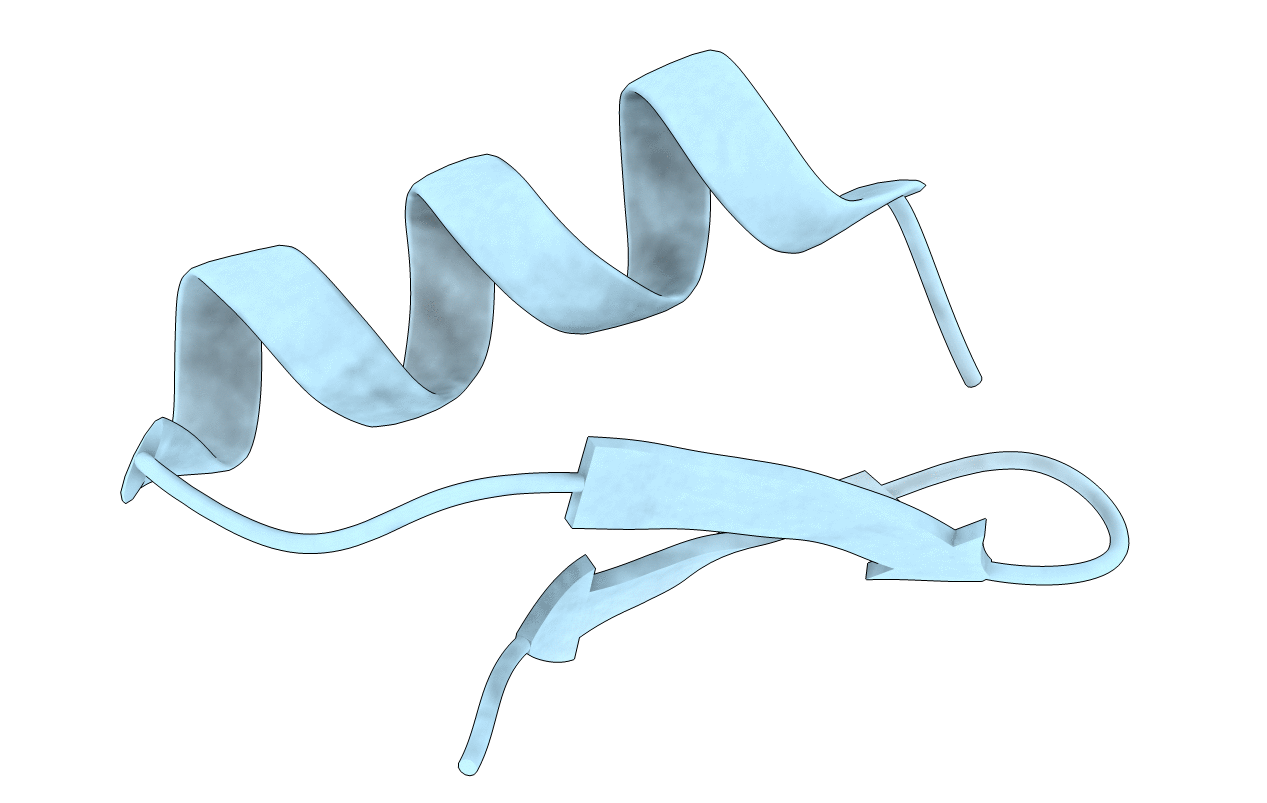
SOLUTION STRUCTURE OF A MINI-PROTEIN REPRODUCING THE CORE OF THE CD4 SURFACE INTERACTING WITH THE HIV-1 ENVELOPE GLYCOPROTEIN
Biological Source:
Source Organism:
Method Details:
Experimental Method:
Conformers Calculated:
40
Conformers Submitted:
1
Selection Criteria:
LOWEST ENERGY


