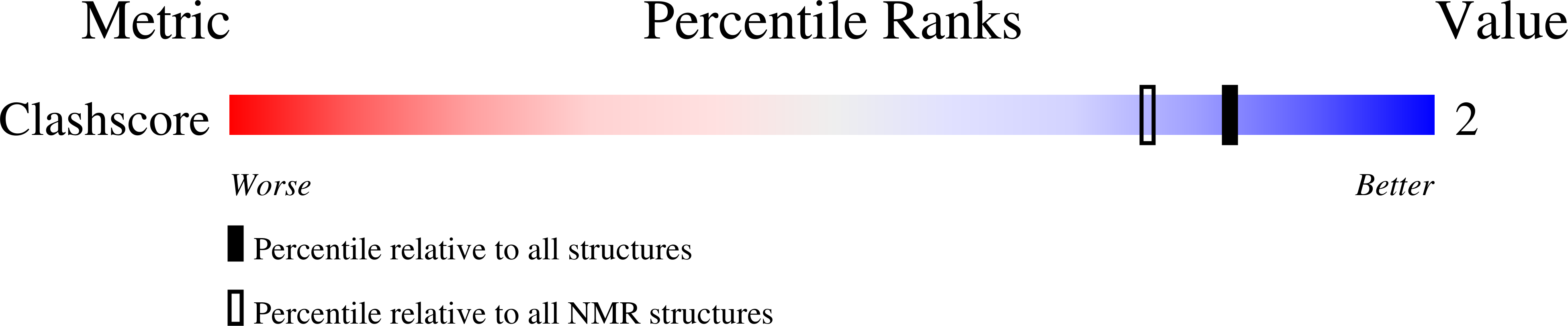Deposition Date
1991-05-15
Release Date
1993-04-15
Last Version Date
2024-05-22
Entry Detail
PDB ID:
1D42
Keywords:
Title:
SOLUTION STRUCTURE OF [D(GTATATAC)]2 VIA RESTRAINED MOLECULAR DYNAMICS SIMULATIONS WITH NUCLEAR MAGNETIC RESONANCE CONSTRAINTS DERIVED FROM RELAXATION MATRIX ANALYSIS OF TWO-DIMENSIONAL NUCLEAR OVERHAUSER EFFECT EXPERIMENTS
Method Details:
Experimental Method:
Conformers Submitted:
5