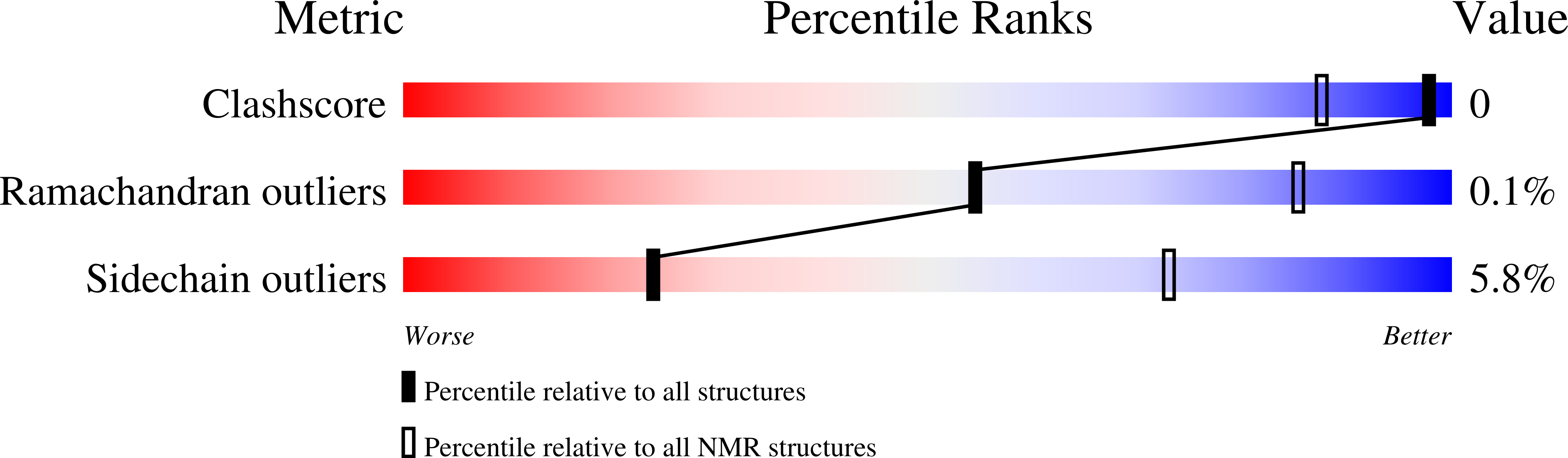
Deposition Date
1999-09-20
Release Date
2000-03-08
Last Version Date
2024-05-22
Entry Detail
PDB ID:
1D1O
Keywords:
Title:
COOPERATIVITY IN EF-HAND CA2+-BINDING PROTEINS: EVIDENCE OF SITE-SITE COMMUNICATION FROM BINDING-INDUCED CHANGES IN STRUCTURE AND DYNAMICS OF N56A CALBINDIN D9K
Biological Source:
Source Organism(s):
Bos taurus (Taxon ID: 9913)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
50
Conformers Submitted:
24
Selection Criteria:
STRUCTURES WITH ACCEPTABLE MOLECULAR ENEGIES WERE ORDERED BY LEAST RESTRAINT VIOLATIONS. THE 24 BEST CONFORMERS WERE SELECTED TO FACILITATE COMPARISON TO PREVIOUS STRUCTURES OF THE PROTEIN AND BECAUSE THIS SURPASSES THE STATISTICAL REQUIREMENT TO REPRESENT ALL OF CONFORMATIONAL SPACE CONSISTENT WITH THE DATA.