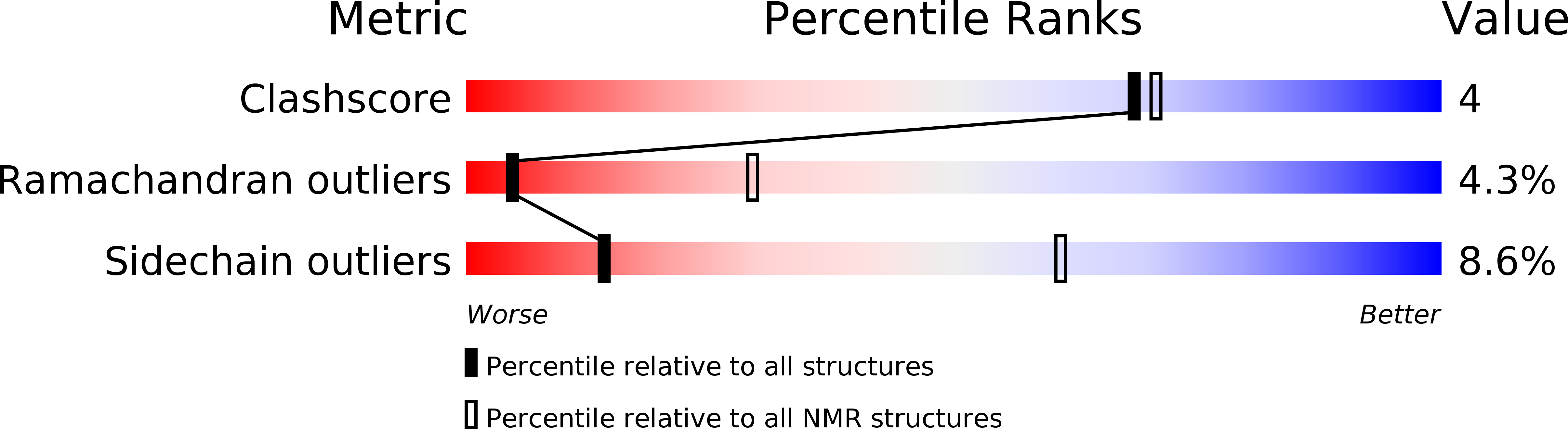
Deposition Date
1999-08-30
Release Date
1999-09-07
Last Version Date
2024-11-20
Entry Detail
PDB ID:
1CXR
Keywords:
Title:
AUTOMATED 2D NOESY ASSIGNMENT AND STRUCTURE CALCULATION OF CRAMBIN(S22/I25) WITH SELF-CORRECTING DISTANCE GEOMETRY BASED NOAH/DIAMOD PROGRAMS
Biological Source:
Source Organism(s):
Crambe hispanica subsp. abyssinica (Taxon ID: 3721)
Method Details:
Experimental Method:
Conformers Calculated:
40
Conformers Submitted:
10
Selection Criteria:
STRUCTURES WITH THE LEAST RESTRAINT VIOLATIONS,STRUCTURES WITH THE LOWEST
ENERGY,TARGET FUNCTION