Deposition Date
1995-07-28
Release Date
1995-12-15
Last Version Date
2024-11-20
Entry Detail
PDB ID:
1CXF
Keywords:
Title:
COMPLEX OF A (D229N/E257Q) DOUBLE MUTANT CGTASE FROM BACILLUS CIRCULANS STRAIN 251 WITH MALTOTETRAOSE AT 120 K AND PH 9.1 OBTAINED AFTER SOAKING THE CRYSTAL WITH ALPHA-CYCLODEXTRIN
Biological Source:
Source Organism(s):
Bacillus circulans (Taxon ID: 1397)
Expression System(s):
Method Details:
Experimental Method:
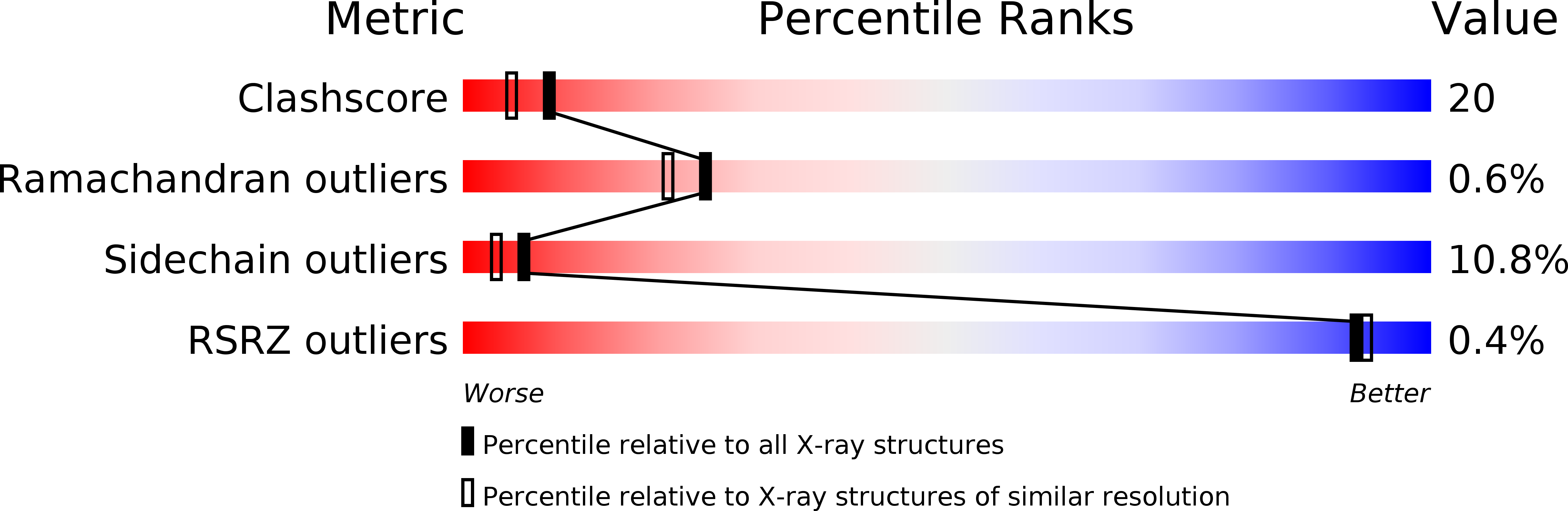
Resolution:
2.10 Å
R-Value Free:
0.2
R-Value Observed:
0.19
Space Group:
P 21 21 21