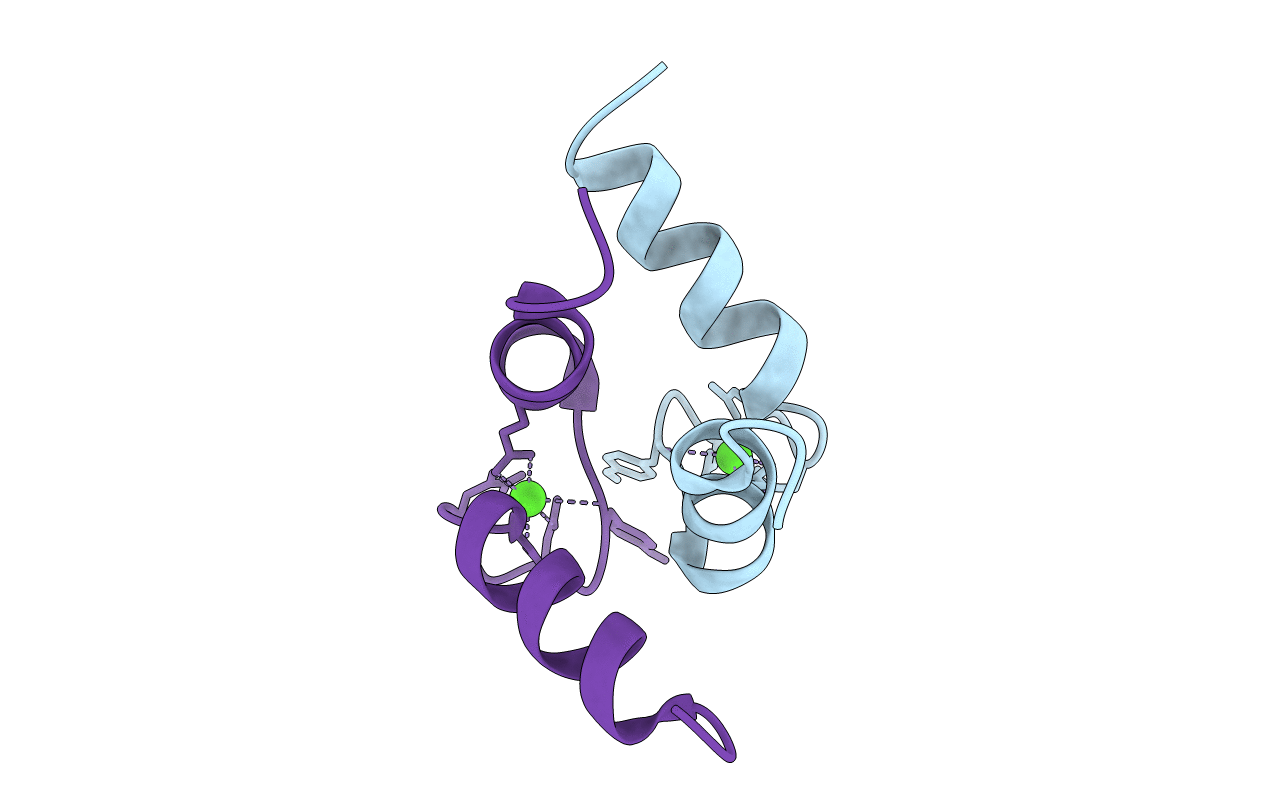
Deposition Date
1992-11-12
Release Date
1993-10-31
Last Version Date
2024-10-23
Entry Detail
PDB ID:
1CTA
Keywords:
Title:
DETERMINATION OF THE SOLUTION STRUCTURE OF A SYNTHETIC TWO-SITE CALCIUM-BINDING HOMODIMERIC PROTEIN DOMAIN BY NMR SPECTROSCOPY
Method Details:
Experimental Method:
Conformers Submitted:
1