Deposition Date
1989-01-13
Release Date
1990-01-15
Last Version Date
2024-05-22
Entry Detail
PDB ID:
1COH
Keywords:
Title:
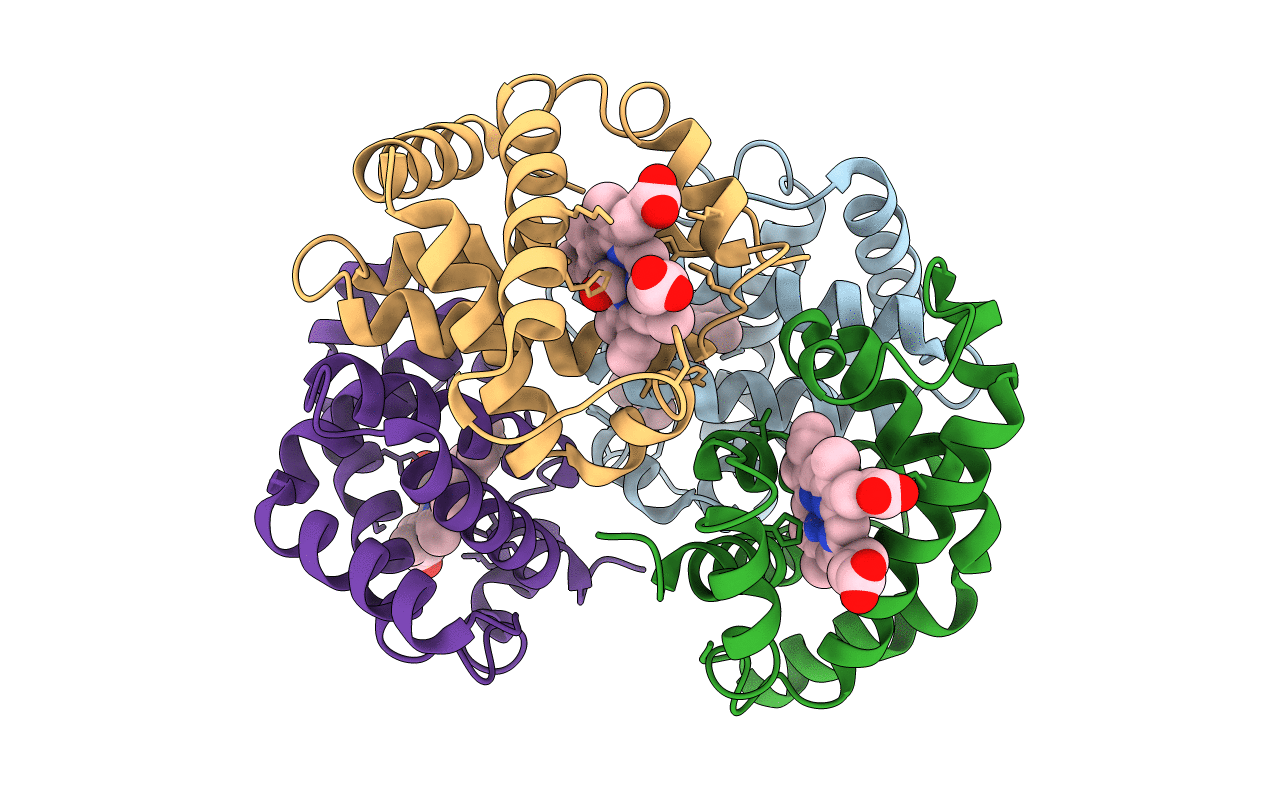
STRUCTURE OF HAEMOGLOBIN IN THE DEOXY QUATERNARY STATE WITH LIGAND BOUND AT THE ALPHA HAEMS
Biological Source:
Source Organism:
Homo sapiens (Taxon ID: 9606)
Method Details: