Deposition Date
1999-05-12
Release Date
1999-07-27
Last Version Date
2023-12-27
Entry Detail
PDB ID:
1CMX
Keywords:
Title:
STRUCTURAL BASIS FOR THE SPECIFICITY OF UBIQUITIN C-TERMINAL HYDROLASES
Method Details:
Experimental Method:
Resolution:
2.25 Å
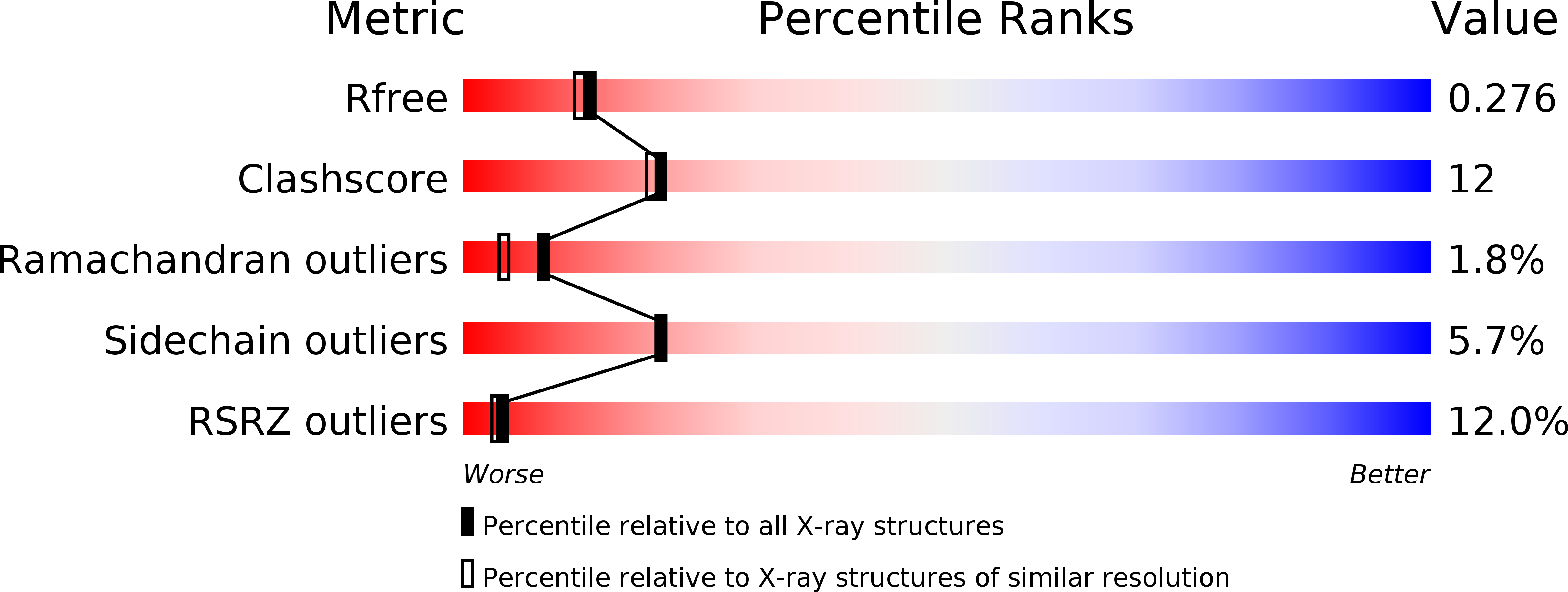
R-Value Free:
0.28
R-Value Work:
0.24
Space Group:
H 3