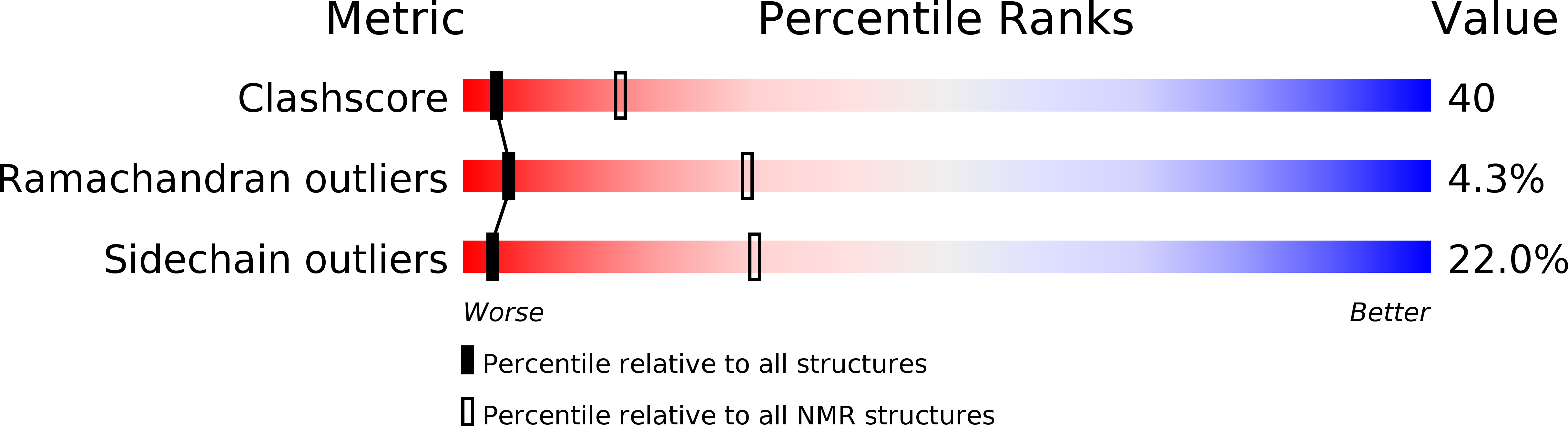Deposition Date
1998-11-20
Release Date
1999-09-10
Last Version Date
2023-12-27
Entry Detail
PDB ID:
1CKK
Keywords:
Title:
CALMODULIN/RAT CA2+/CALMODULIN DEPENDENT PROTEIN KINASE FRAGMENT
Biological Source:
Source Organism(s):
Xenopus laevis (Taxon ID: 8355)
Rattus norvegicus (Taxon ID: 10116)
Rattus norvegicus (Taxon ID: 10116)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
30
Selection Criteria:
LOWEST ENERGY