Deposition Date
1993-10-20
Release Date
1994-01-31
Last Version Date
2024-02-07
Entry Detail
PDB ID:
1CIM
Keywords:
Title:
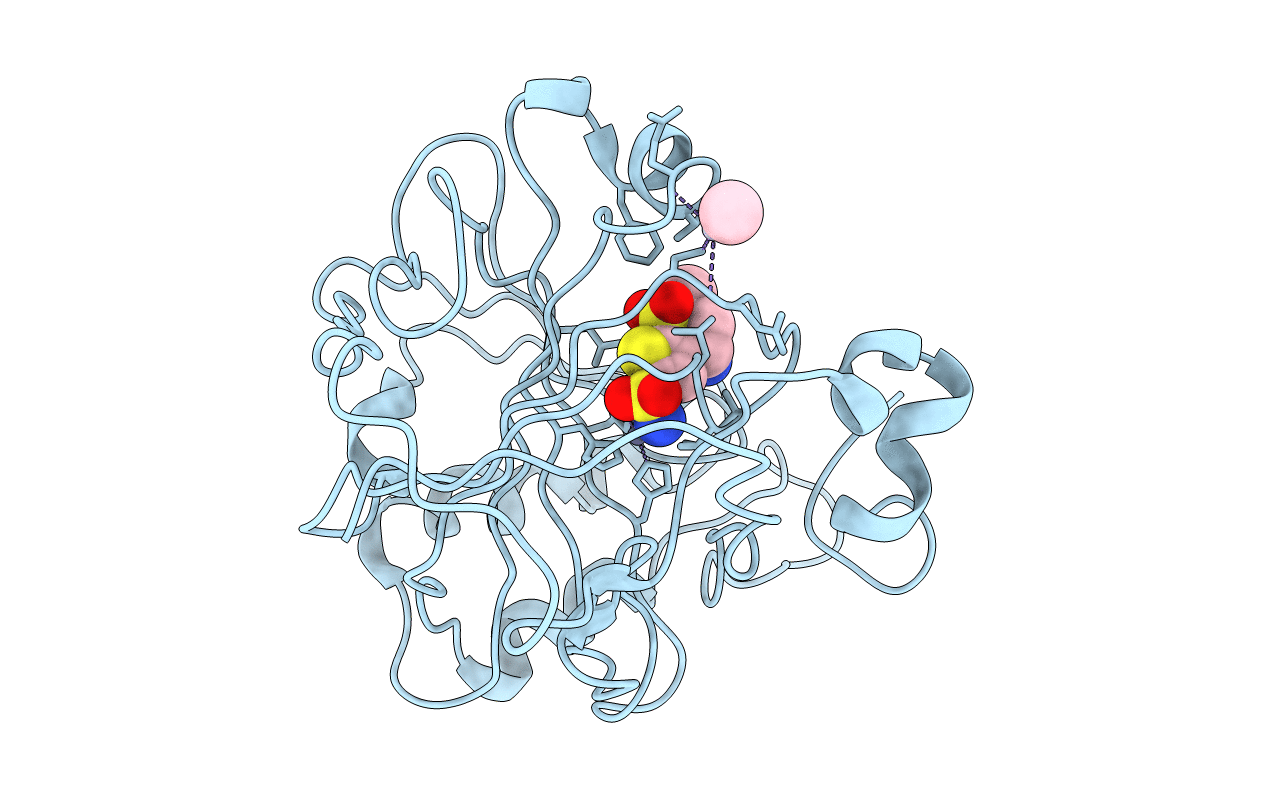
THE POSITIONS OF HIS-64 AND A BOUND WATER IN HUMAN CARBONIC ANHYDRASE II UPON BINDING THREE STRUCTURALLY RELATED INHIBITORS
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Method Details: