Deposition Date
1999-03-19
Release Date
1999-06-25
Last Version Date
2024-10-30
Entry Detail
PDB ID:
1CFJ
Keywords:
Title:
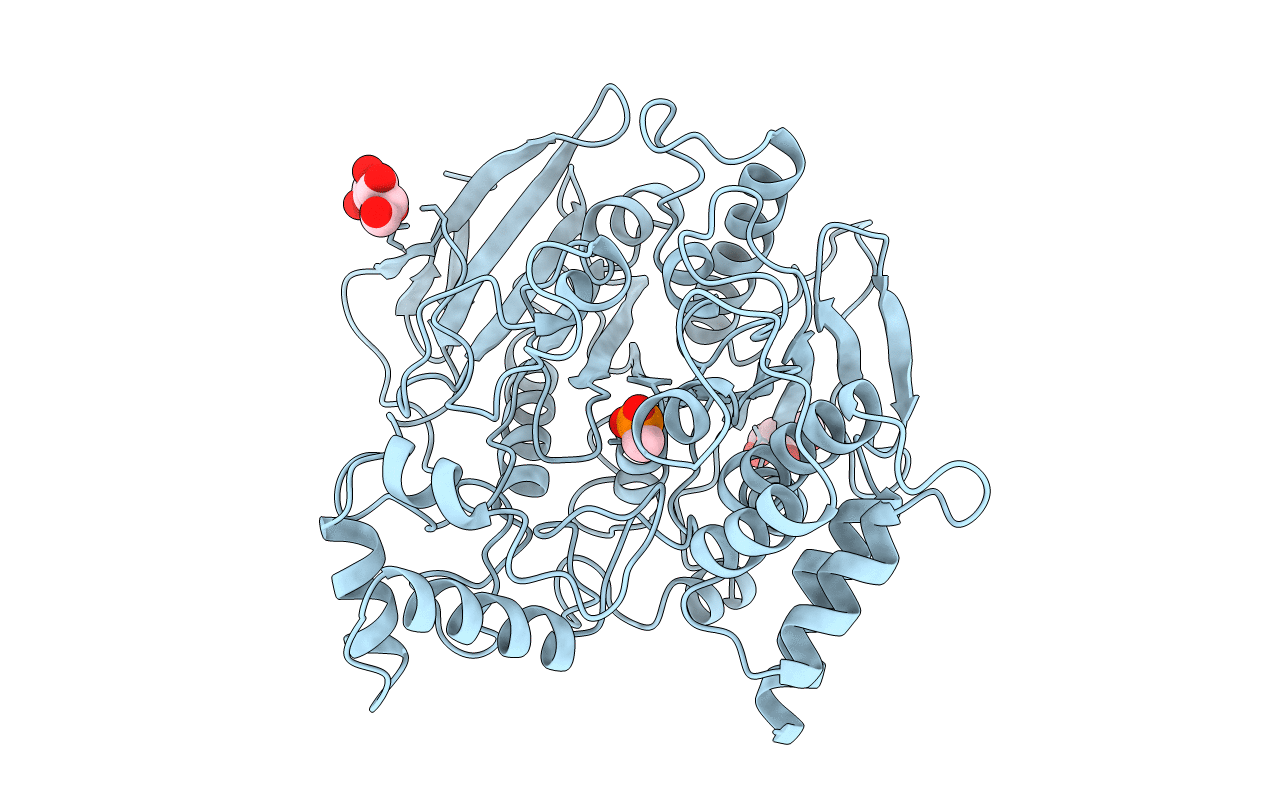
METHYLPHOSPHONYLATED ACETYLCHOLINESTERASE (AGED) OBTAINED BY REACTION WITH O-ISOPROPYLMETHYLPHOSPHONOFLUORIDATE (GB, SARIN)
Biological Source:
Source Organism(s):
Torpedo californica (Taxon ID: 7787)
Method Details:
Experimental Method:
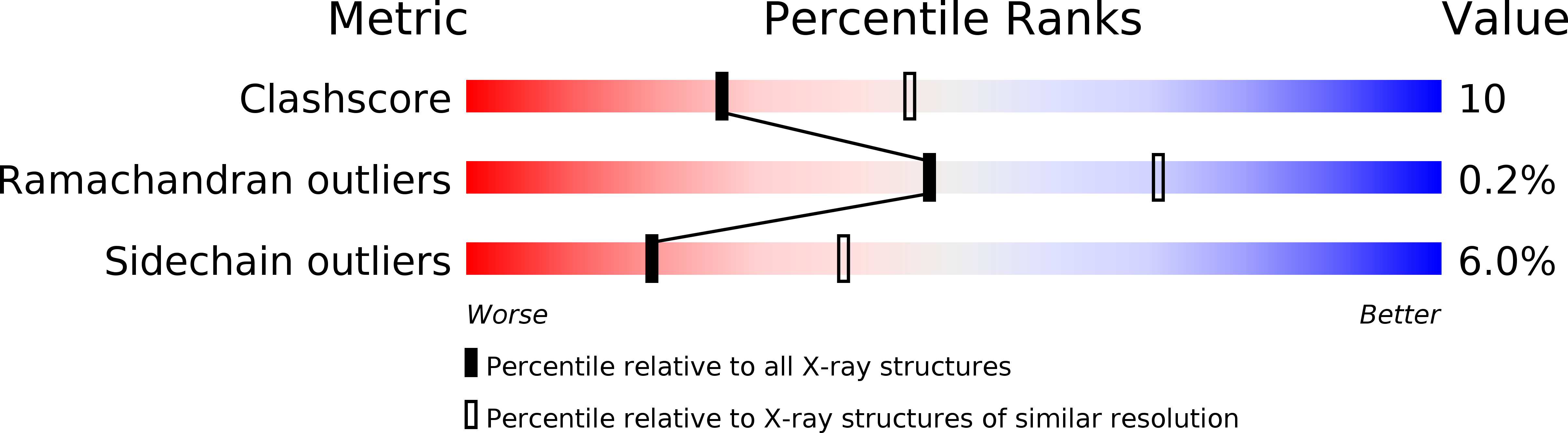
Resolution:
2.60 Å
R-Value Free:
0.24
R-Value Work:
0.18
Space Group:
P 31 2 1