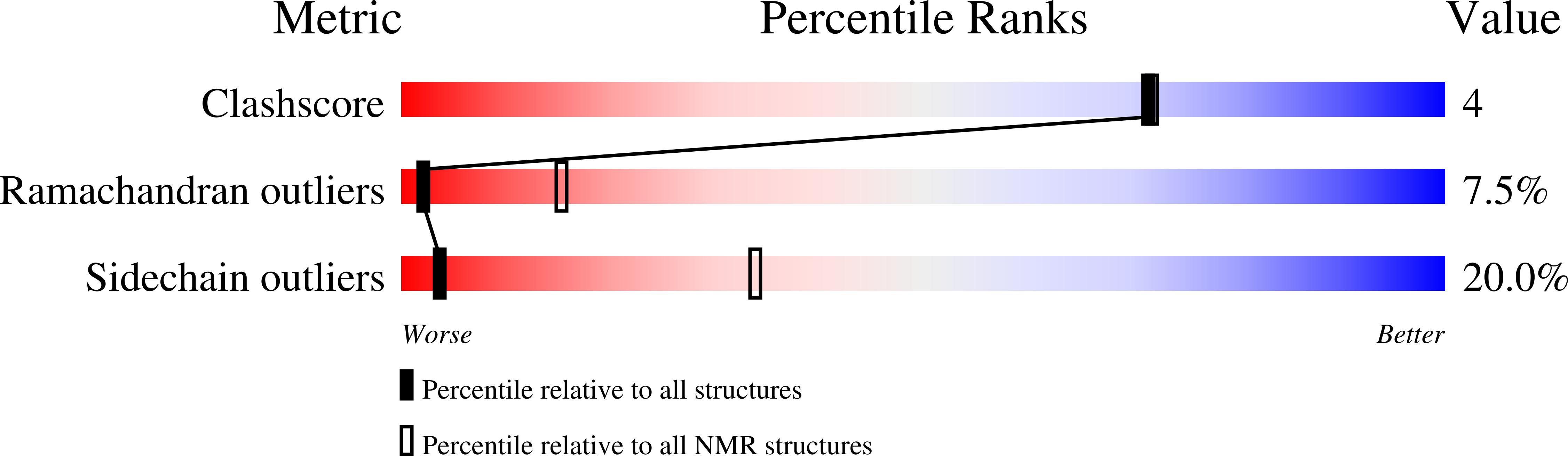
Deposition Date
1999-03-14
Release Date
1999-03-27
Last Version Date
2024-10-30
Entry Detail
PDB ID:
1CE3
Keywords:
Title:
PUTATIVE ANCESTRAL PROTEIN ENCODED BY A SINGLE SEQUENCE REPEAT OF THE MULTIDOMAIN PROTEINASE INHIBITOR FROM NICOTIANA ALATA
Biological Source:
Source Organism(s):
Nicotiana alata (Taxon ID: 4087)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
50
Conformers Submitted:
20
Selection Criteria:
LEAST RESTRAINT VIOLATIONS AND LOWEST ENERGY