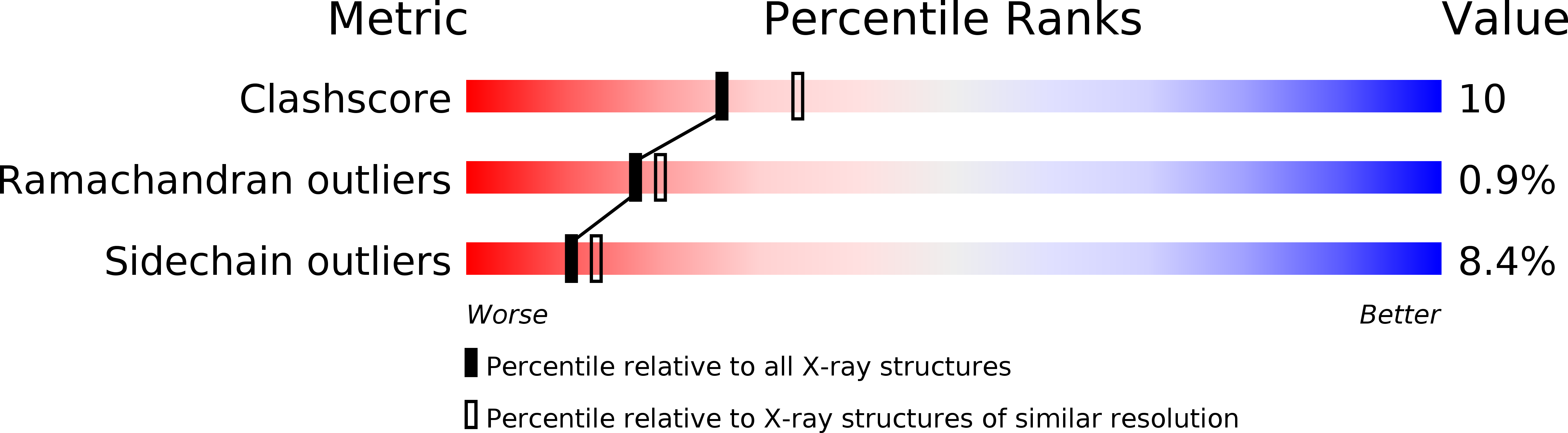Deposition Date
2000-05-16
Release Date
2000-05-31
Last Version Date
2023-08-09
Entry Detail
PDB ID:
1C8L
Keywords:
Title:
SYNERGISTIC INHIBITION OF GLYCOGEN PHOSPHORYLASE A BY A POTENTIAL ANTIDIABETIC DRUG AND CAFFEINE
Biological Source:
Source Organism(s):
Oryctolagus cuniculus (Taxon ID: 9986)
Method Details:
Experimental Method:
Resolution:
2.30 Å
R-Value Free:
0.25
R-Value Work:
0.21
R-Value Observed:
0.21
Space Group:
P 43 21 2