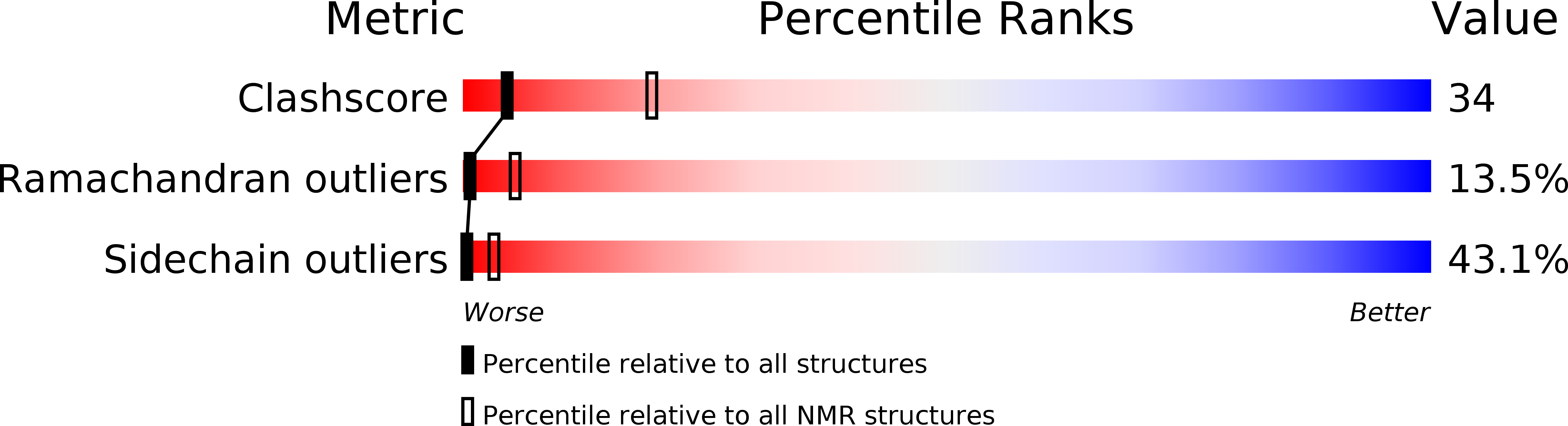
Deposition Date
1990-05-14
Release Date
1991-04-15
Last Version Date
2024-10-16
Entry Detail
PDB ID:
1BUS
Keywords:
Title:
SOLUTION CONFORMATION OF PROTEINASE INHIBITOR IIA FROM BULL SEMINAL PLASMA BY 1H NUCLEAR MAGNETIC RESONANCE AND DISTANCE GEOMETRY
Biological Source:
Source Organism(s):
Bos taurus (Taxon ID: 9913)
Method Details:
Experimental Method:
Conformers Submitted:
5