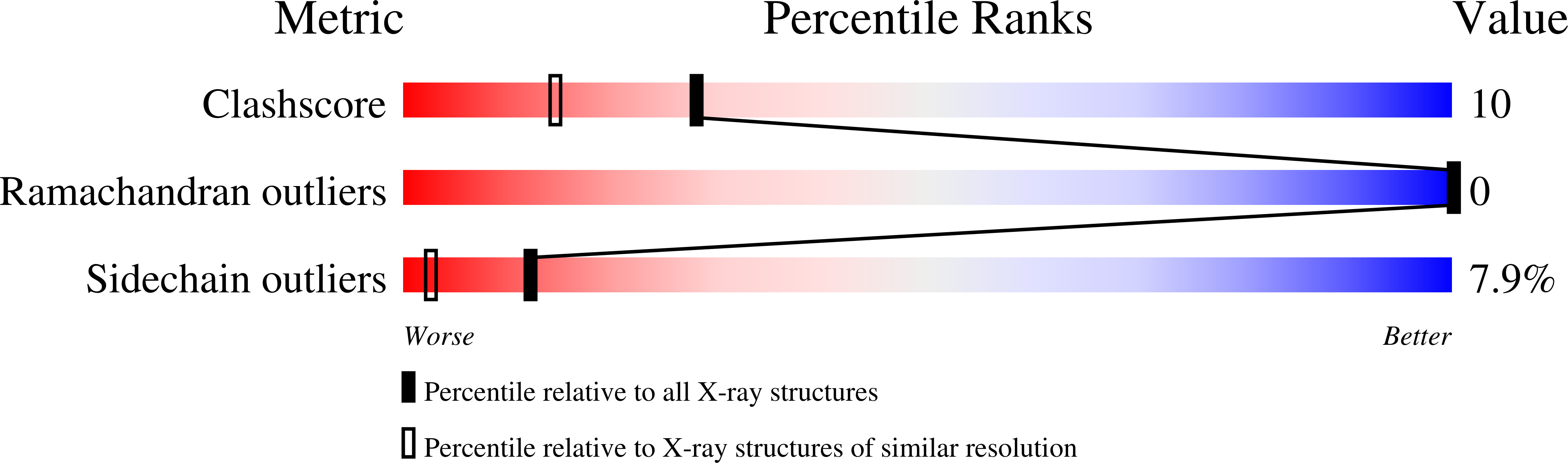
Deposition Date
1998-09-02
Release Date
1998-12-30
Last Version Date
2023-08-09
Entry Detail
Biological Source:
Source Organism:
Arabidopsis thaliana (Taxon ID: 3702)
Host Organism:
Method Details: