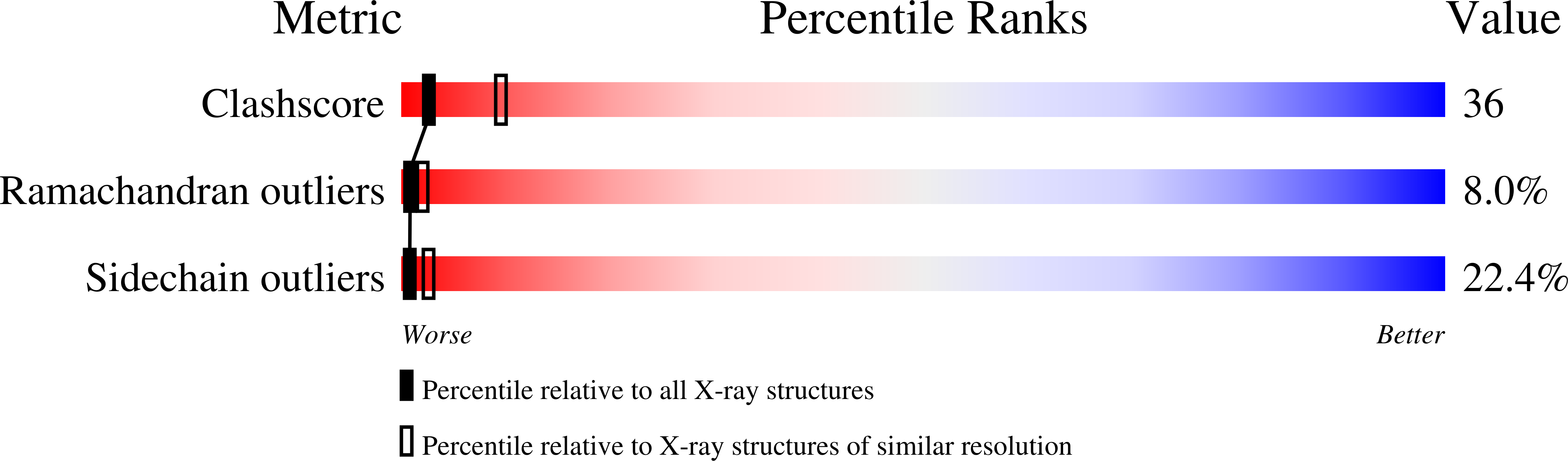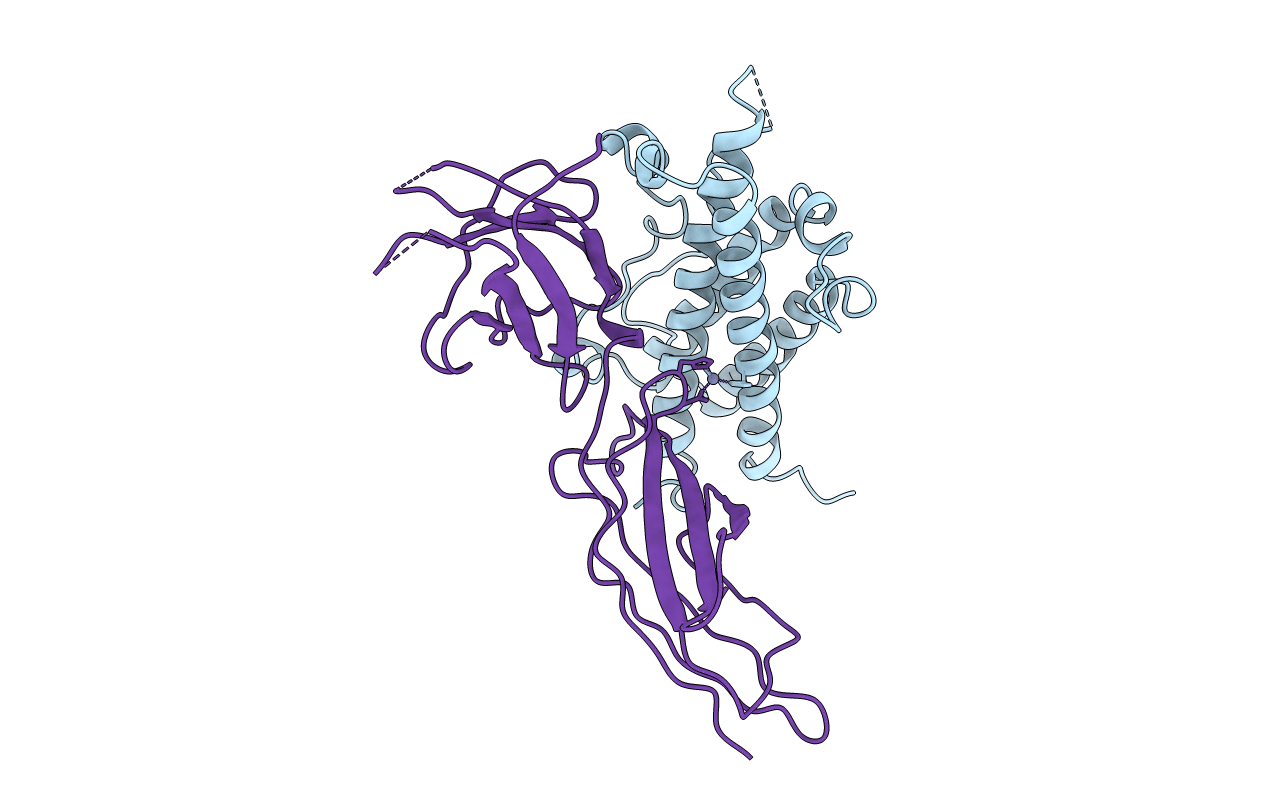
Deposition Date
1998-08-12
Release Date
1998-08-19
Last Version Date
2024-11-13
Entry Detail
PDB ID:
1BP3
Keywords:
Title:
THE XRAY STRUCTURE OF A GROWTH HORMONE-PROLACTIN RECEPTOR COMPLEX
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details: