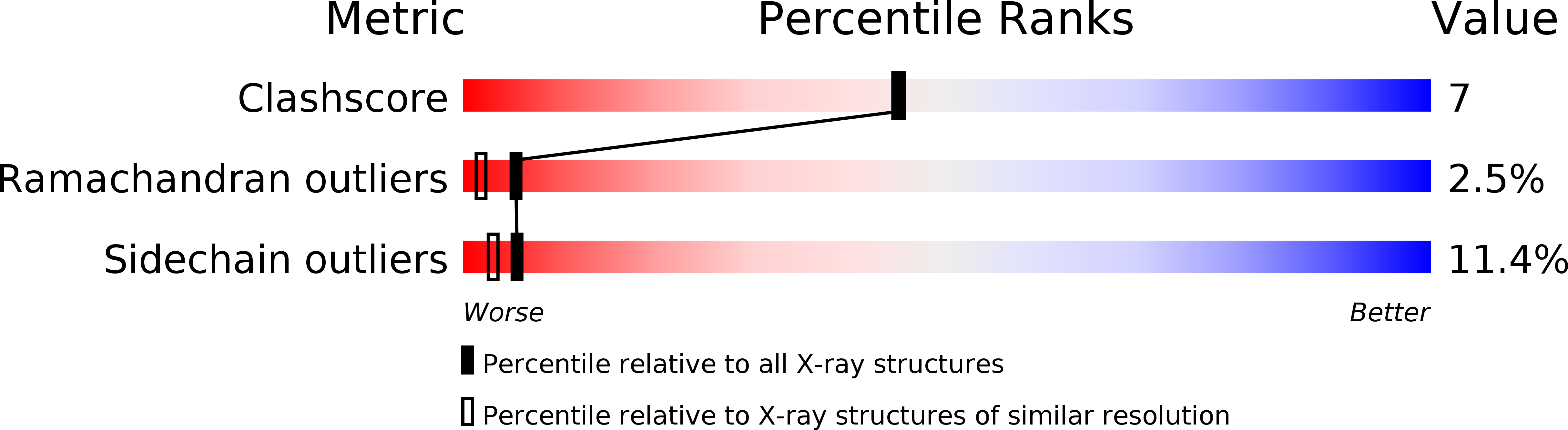Deposition Date
1998-07-29
Release Date
1999-01-06
Last Version Date
2024-02-07
Entry Detail
PDB ID:
1BM9
Keywords:
Title:
REPLICATION TERMINATOR PROTEIN FROM BACILLUS SUBTILIS
Biological Source:
Source Organism:
Bacillus subtilis (Taxon ID: 1423)
Host Organism:
Method Details:
Experimental Method:
Resolution:
2.00 Å
R-Value Free:
0.28
R-Value Work:
0.20
R-Value Observed:
0.20
Space Group:
C 1 2 1