Abstact
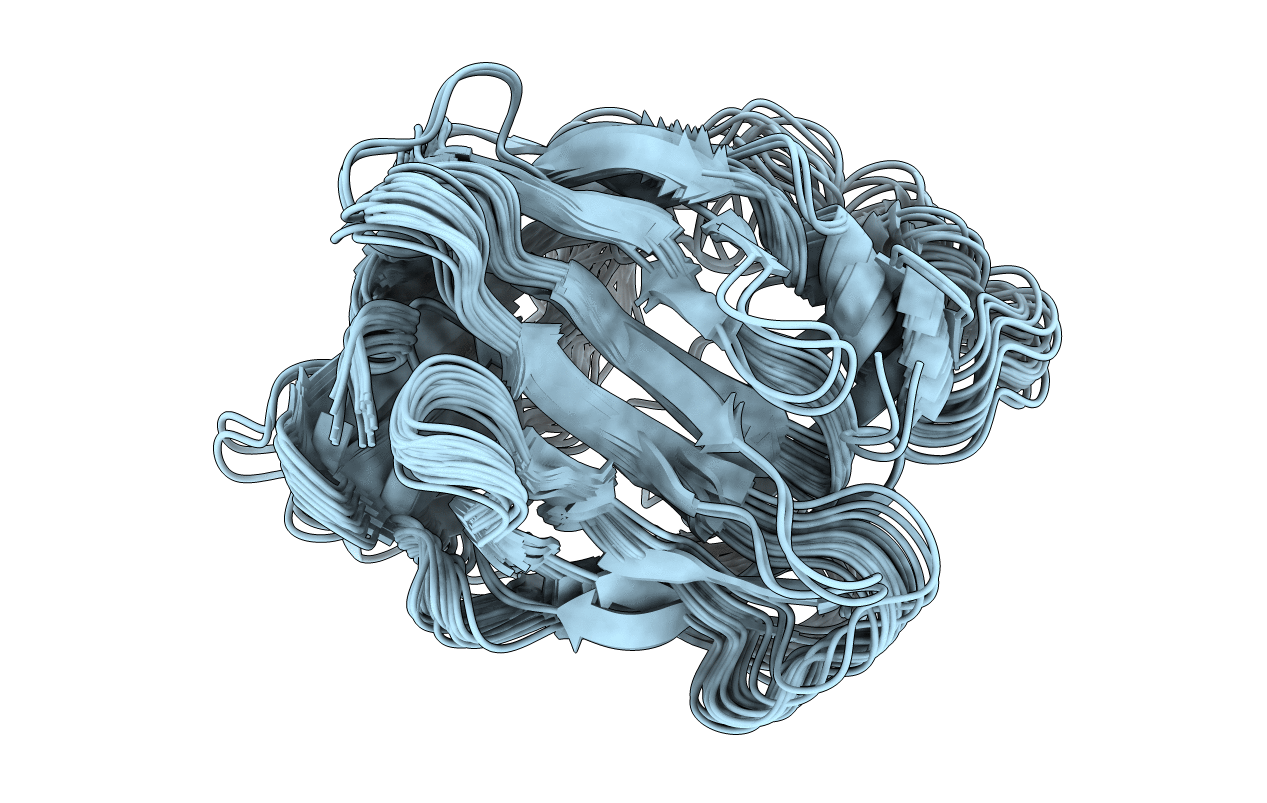
The structure of human apo-cellular retinoic acid binding protein II (apo-CRABPII) in solution at pH 7.3 has been determined by NMR spectroscopy. The sequential assignments of the 1H, 13C, and 15N resonances of apo-CRABPII were established by multinuclear, multidimensional NMR spectroscopy. The solution structure of apo-CRABPII was derived from 2382 experimental NMR restraints using a hybrid distance geometry-simulated annealing protocol. The root-mean-square deviation of the ensemble of 25 refined conformers that represent the structure from the mean coordinate set derived from them was 0.54 +/- 0.18 and 0.92 +/- 0.20 A for the backbone atoms and all heavy atoms, respectively, of all residues except Ala32-Pro39 and Thr57-Glu62, which are in disordered regions. The solution structure of apo-CRABPII is similar to the crystal structure of holo-CRABPII [Kleywegt, G. J., Bergfors, T., Senn, H., Le Motte, P., Gsell, B., Shudo, K., and Jones, T. A. (1994) Structure 2, 1241-1258] except the ligand entrance, which is sufficiently enlarged in the apoprotein to be readily accessible to retinoic acid. The enlargement of the ligand entrance of apo-CRABPII relative to that of holo-CRABPII is due mainly to a concerted conformational change in three structural elements, namely, the second helix, the betaC-betaD loop, and the betaE-betaF loop. Furthermore, the ligand-binding pocket of apo-CRABPII showed evidence of dynamic disorder; among the 21 residues that constitute this pocket, 16 residues had weak or no detectable cross-peaks in the two-dimensional 1H-15N HSQC spectrum recorded under conditions of minimal water saturation or dephasing. Apo-CRABPII is largely monomeric in solution, with no evidence for the dimeric structure shown in the crystal structure of apo-CRABPI which was suggested to be a prerequisite for ligand entry [Thompson, J. R., Bratt, J. M., and Banaszak, L. J. (1995) J. Mol. Biol. 252, 433-446]. Thus, the widening of the ligand entrance required for entry of retinoic acid appears to be a property of monomeric apo-CRABPII.