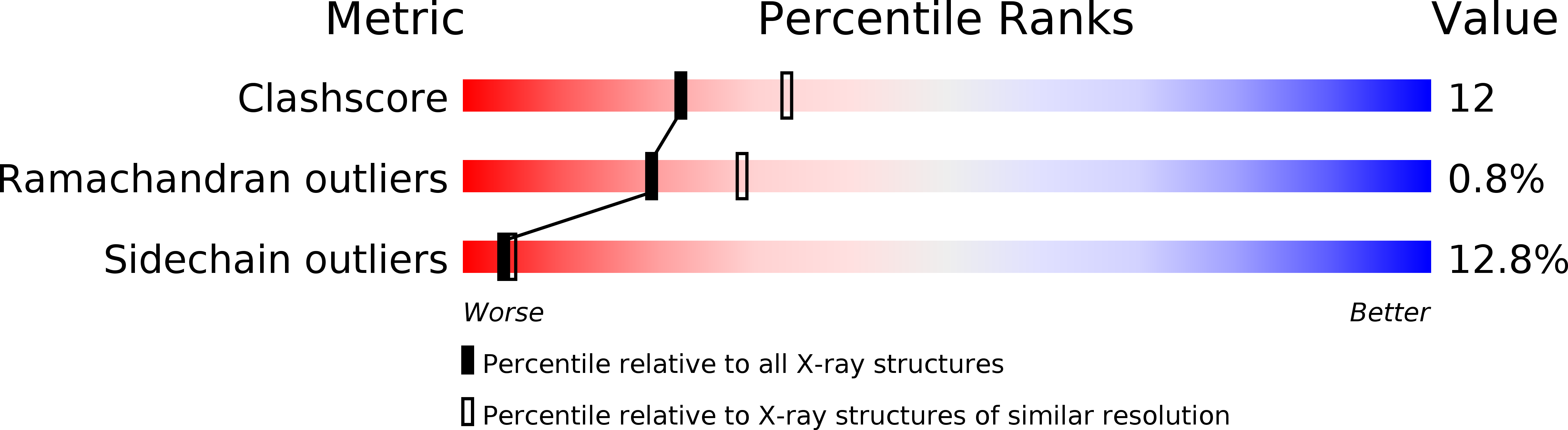
Deposition Date
1993-03-02
Release Date
1994-01-31
Last Version Date
2024-10-30
Entry Detail
PDB ID:
1BLL
Keywords:
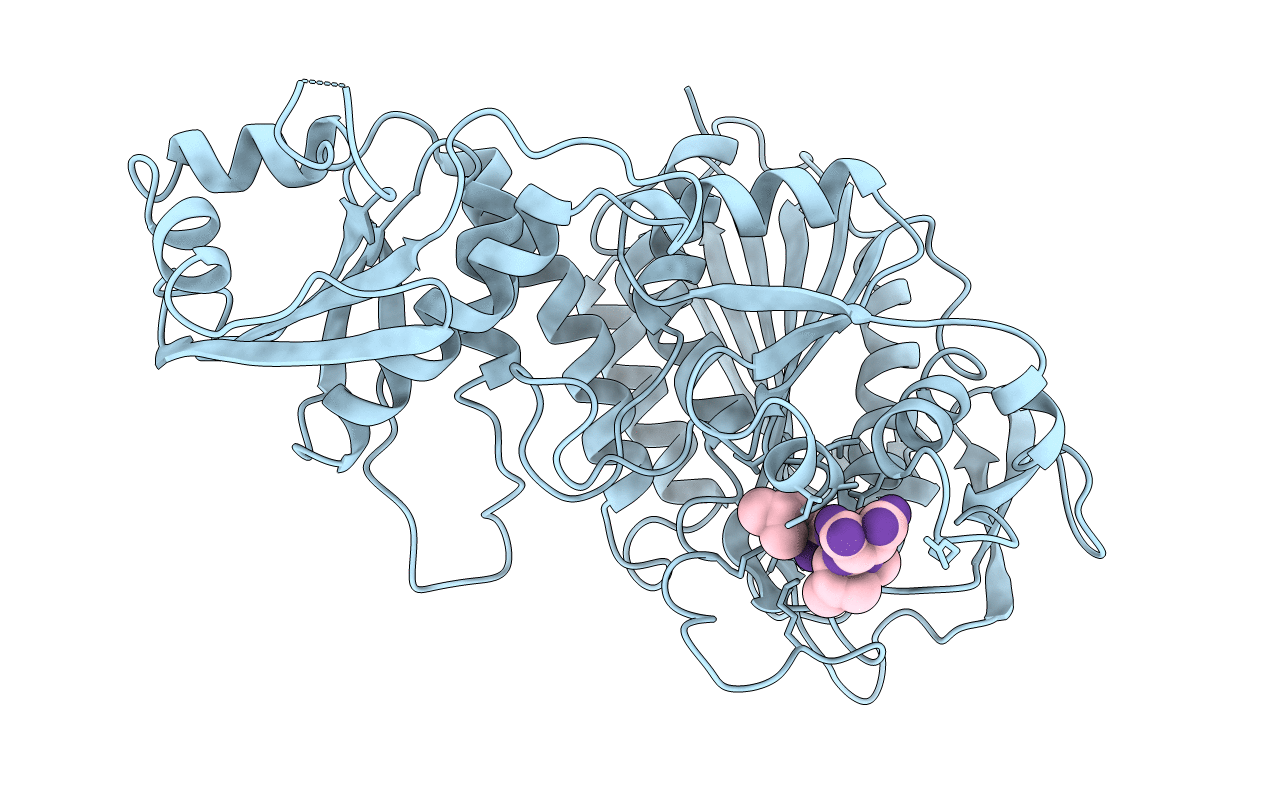
Title:
X-RAY CRYSTALLOGRAPHIC DETERMINATION OF THE STRUCTURE OF BOVINE LENS LEUCINE AMINOPEPTIDASE COMPLEXED WITH AMASTATIN: FORMULATION OF A CATALYTIC MECHANISM FEATURING A GEM-DIOLATE TRANSITION STATE
Biological Source:
Source Organism(s):
Bos taurus (Taxon ID: 9913)
Streptomyces sp. ME98-M3 (Taxon ID: )
Streptomyces sp. ME98-M3 (Taxon ID: )
Method Details:
Experimental Method:
Resolution:
2.40 Å
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 63 2 2