Abstact
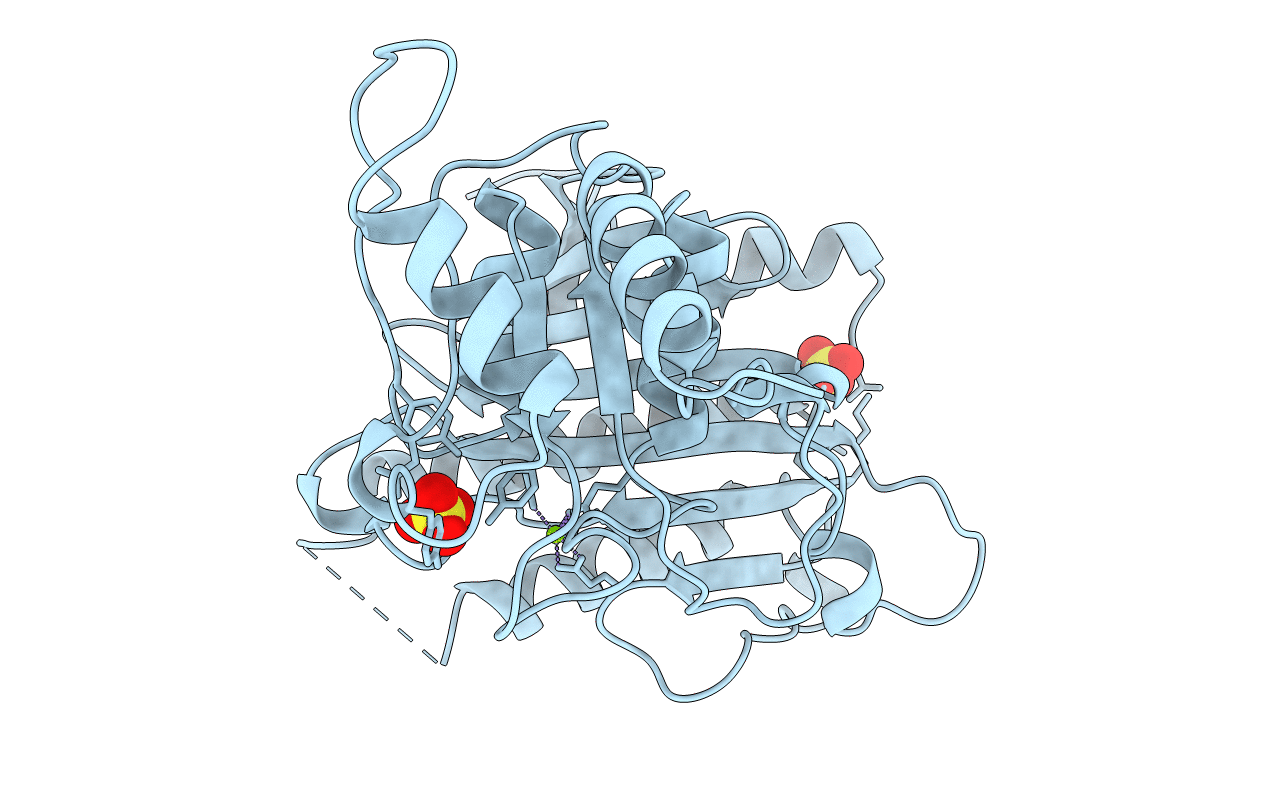
The three-dimensional structure of the R form of rabbit liver fructose 1,6-bisphosphatase (Fru-1,6-Pase; E.C. 3.1.3.11) has been determined by a combination of heavy-atom and molecular-replacement methods. A model, which includes 2394 protein atoms and 86 water molecules, has been refined at 2.3 A resolution to a crystallographic R factor of 0.177. The root-mean-square deviations of bond distances and angles from standard geometry are 0.012 A and 1.7 degrees, respectively. This structural result, in conjunction with recently redetermined amino-acid sequence data, unequivocally establishes that the rabbit liver enzyme is not an aberrant bisphosphatase as once believed, but is indeed homologous to other Fru-1,6-Pases. The root-mean-square deviation of the Calpha atoms in the rabbit liver structure from the homologous atoms in the pig kidney structure complexed with the product, fructose 6-phosphate, is 0.7 A. Fru-1,6-Pases are homotetramers, and the rabbit liver protein crystallizes in space group I222 with one monomer in the asymmetric unit. The structure contains a single endogenous Mg2+ ion coordinated by Glu97, Asp118, Asp121 and Glu280 at the site designated metal site 1 in pig kidney Fru-1,6-Pase R-form complexes. In addition, two sulfate ions, which are found at the positions normally occupied by the 6-phosphate group of the substrate, as well as the phosphate of the allosteric inhibitor AMP appear to provide stability. Met177, which has hydrophobic contacts with the adenine moiety of AMP in pig kidney T-form complexes, is replaced by glycine. Binding of a non-hydrolyzable substrate analog, beta-methyl-fructose 1,6-bisphosphate, at the catalytic site is also examined.