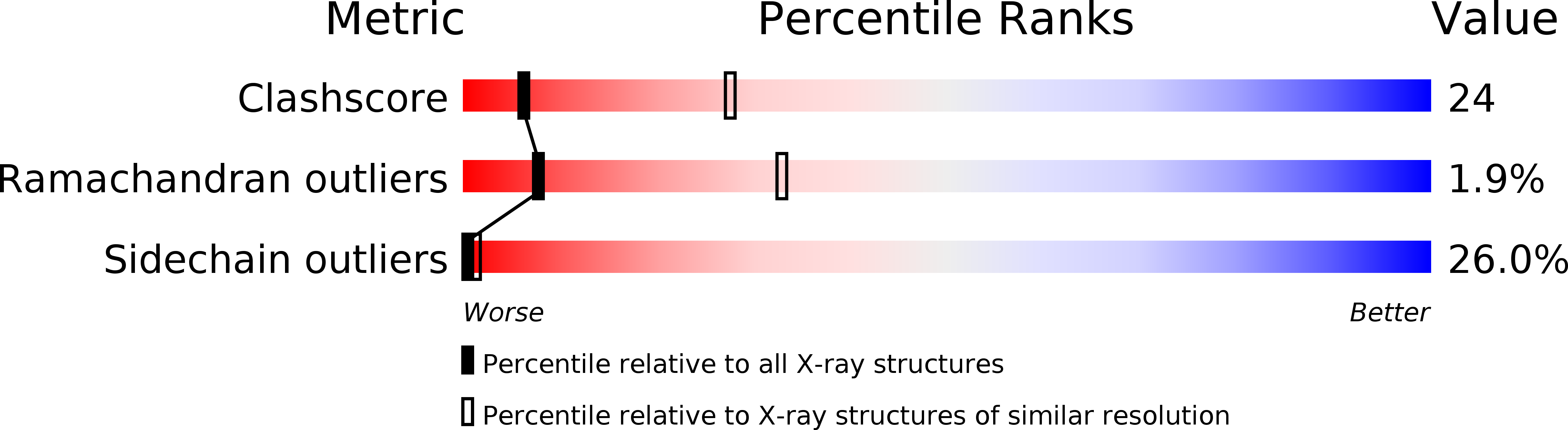Deposition Date
1998-06-17
Release Date
1998-10-14
Last Version Date
2024-11-13
Entry Detail
PDB ID:
1BIH
Keywords:
Title:
CRYSTAL STRUCTURE OF THE INSECT IMMUNE PROTEIN HEMOLIN: A NEW DOMAIN ARRANGEMENT WITH IMPLICATIONS FOR HOMOPHILIC ADHESION
Biological Source:
Source Organism:
Hyalophora cecropia (Taxon ID: 7123)
Host Organism:
Method Details:
Experimental Method:
Resolution:
3.10 Å
R-Value Free:
0.26
R-Value Work:
0.21
R-Value Observed:
0.21
Space Group:
P 21 21 21