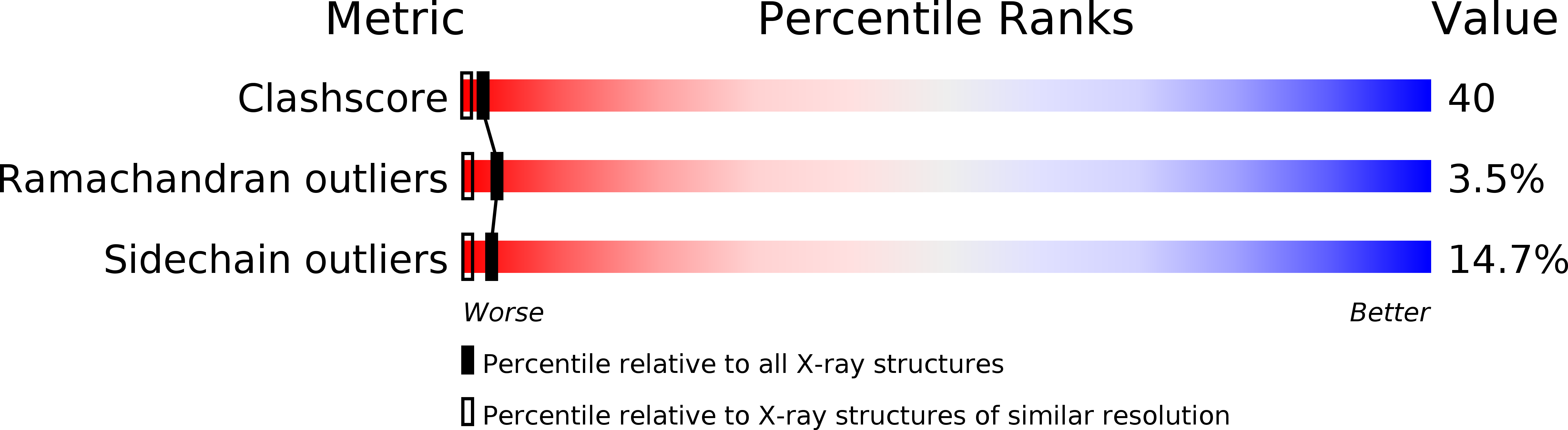Deposition Date
1998-05-13
Release Date
1998-08-12
Last Version Date
2024-10-30
Entry Detail
PDB ID:
1BEA
Keywords:
Title:
BIFUNCTIONAL HAGEMAN FACTOR/AMYLASE INHIBITOR FROM MAIZE
Biological Source:
Method Details:
Experimental Method:
Resolution:
1.95 Å
R-Value Free:
0.28
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 42 21 2