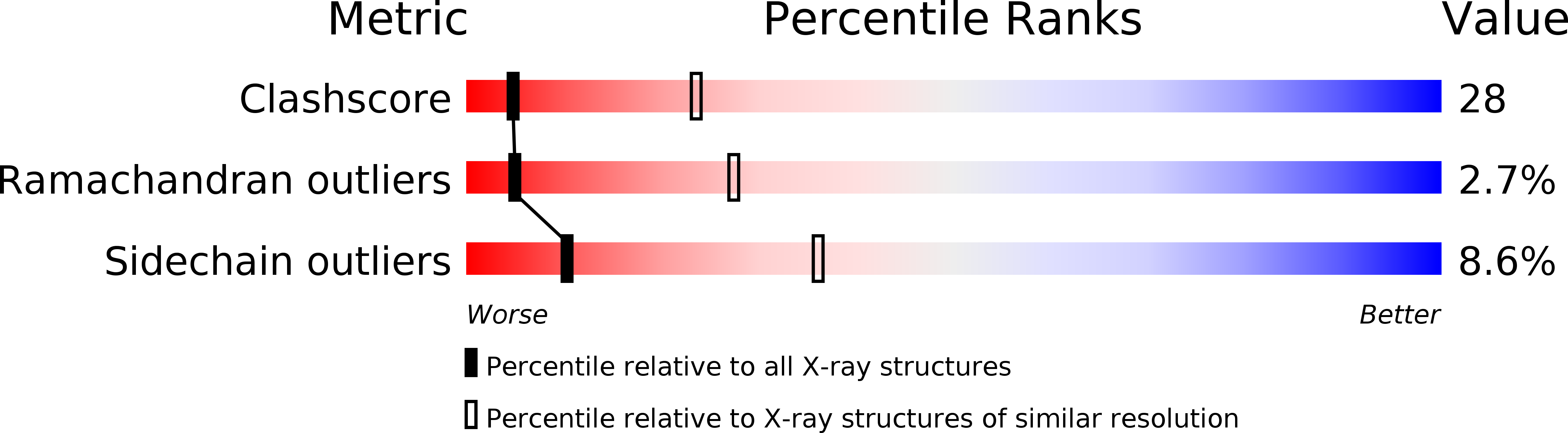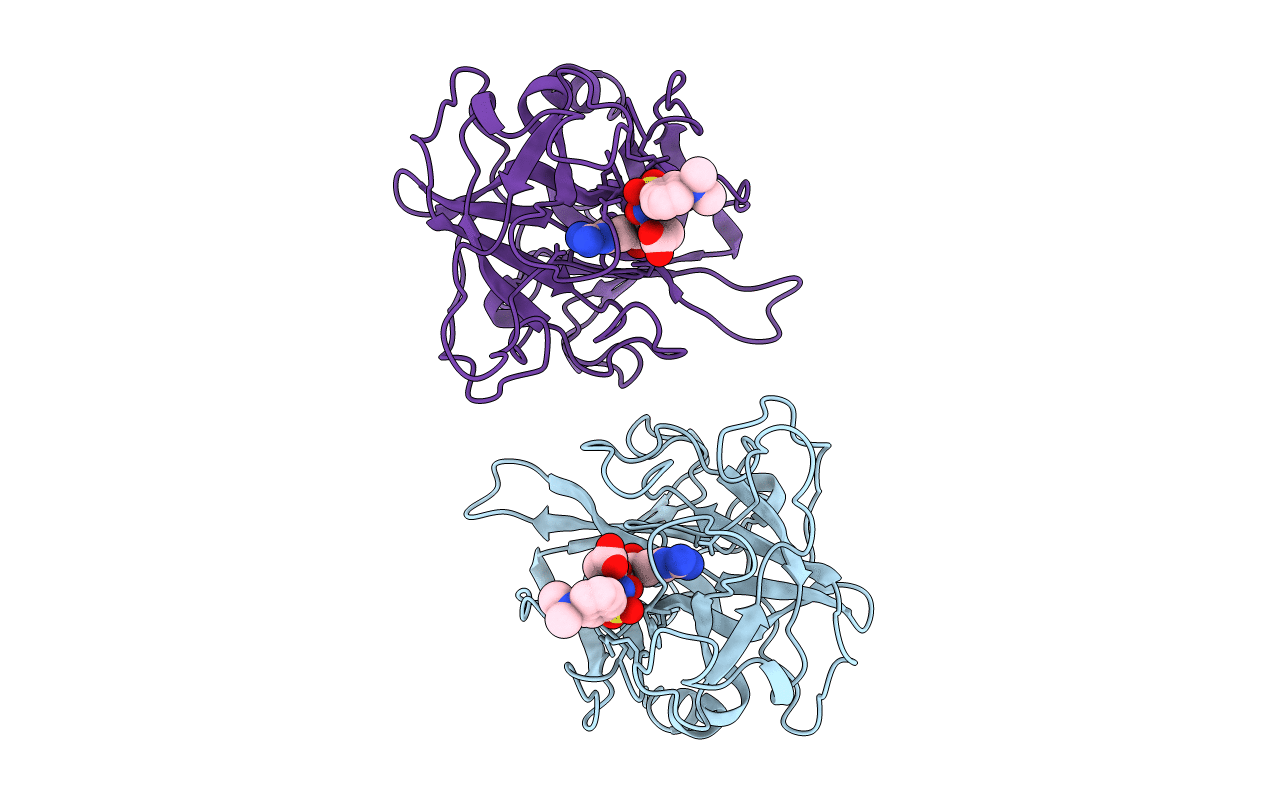
Deposition Date
1998-05-07
Release Date
1999-05-11
Last Version Date
2024-10-16
Entry Detail
PDB ID:
1BDA
Keywords:
Title:
CATALYTIC DOMAIN OF HUMAN SINGLE CHAIN TISSUE PLASMINOGEN ACTIVATOR IN COMPLEX WITH DANSYL-EGR-CMK (DANSYL-GLU-GLY-ARG CHLOROMETHYL KETONE)
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details: