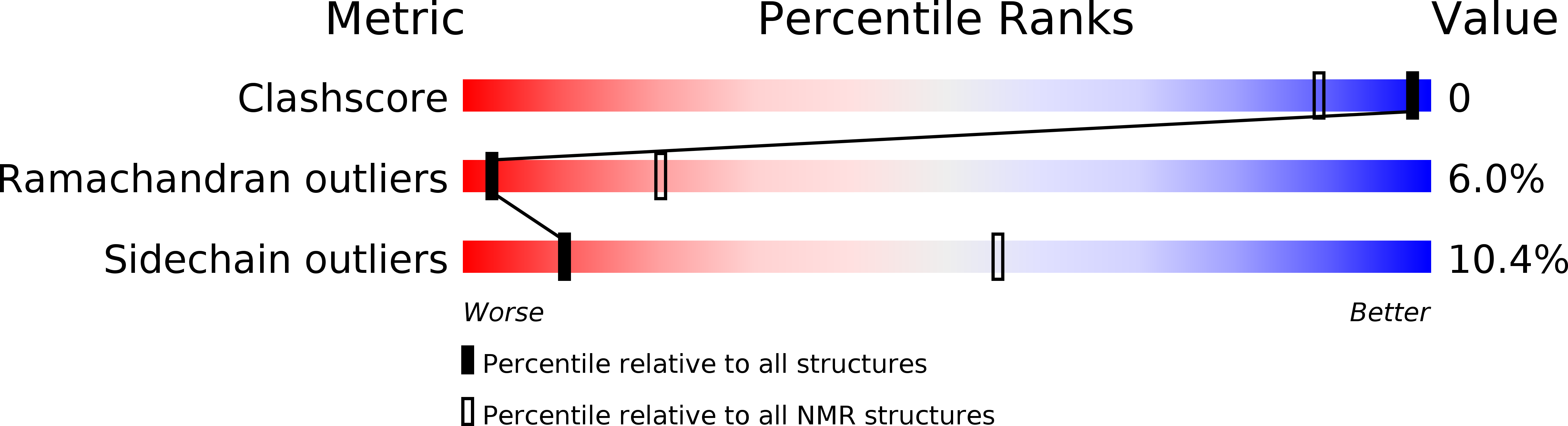
Deposition Date
1996-06-06
Release Date
1997-01-11
Last Version Date
2025-03-26
Entry Detail
PDB ID:
1BAH
Keywords:
Title:
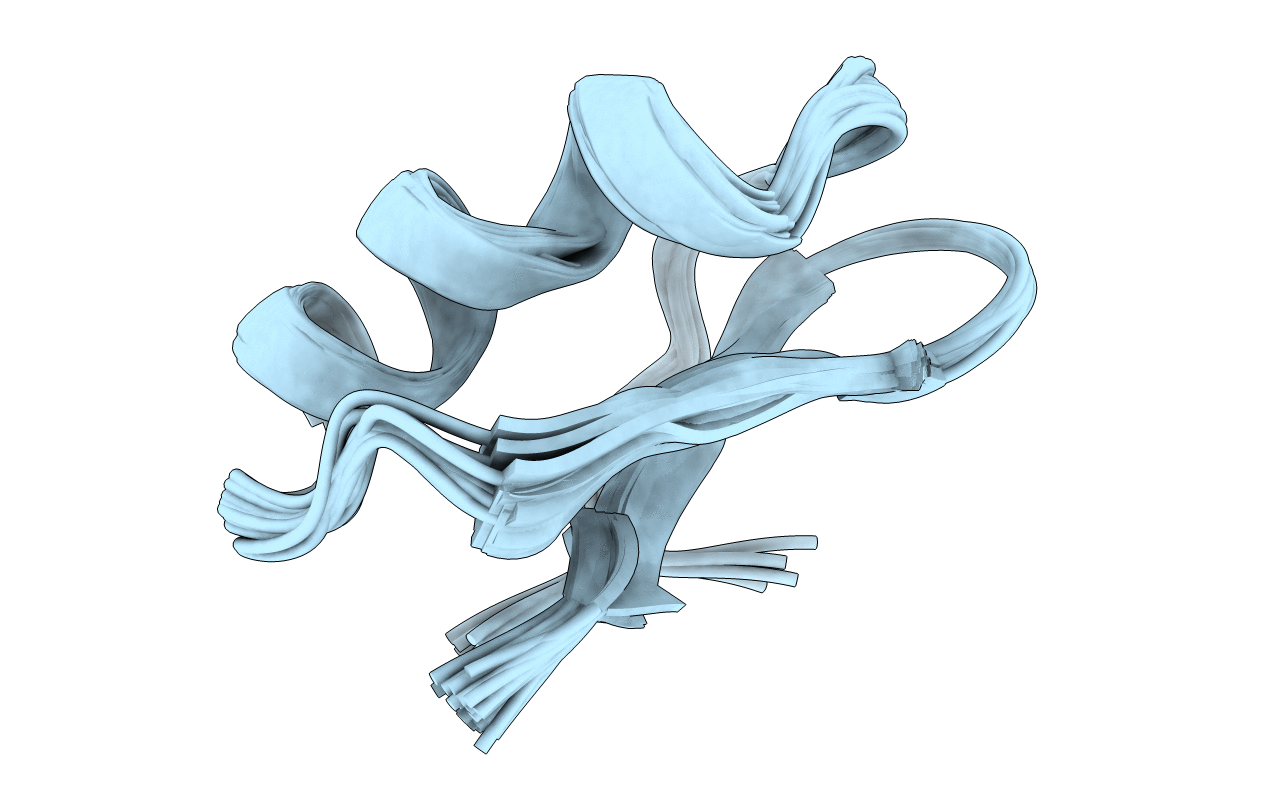
A TWO DISULFIDE DERIVATIVE OF CHARYBDOTOXIN WITH DISULFIDE 13-33 REPLACED BY TWO ALPHA-AMINOBUTYRIC ACIDS, NMR, 30 STRUCTURES
Biological Source:
Source Organism(s):
Leiurus quinquestriatus (Taxon ID: 6883)
Method Details:
Experimental Method:
Conformers Submitted:
30