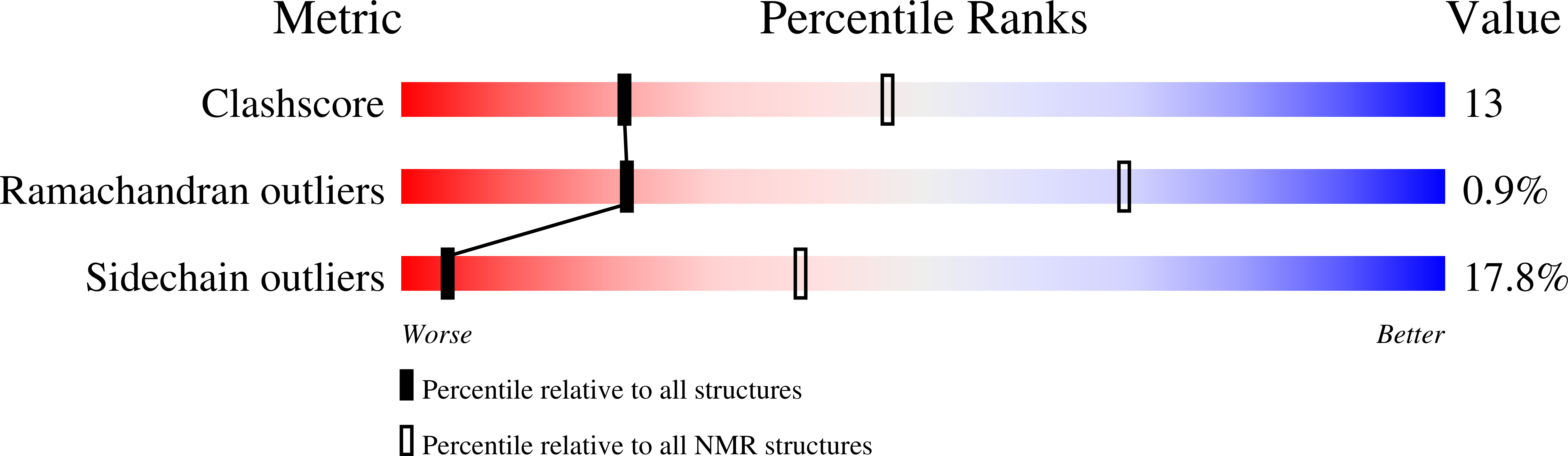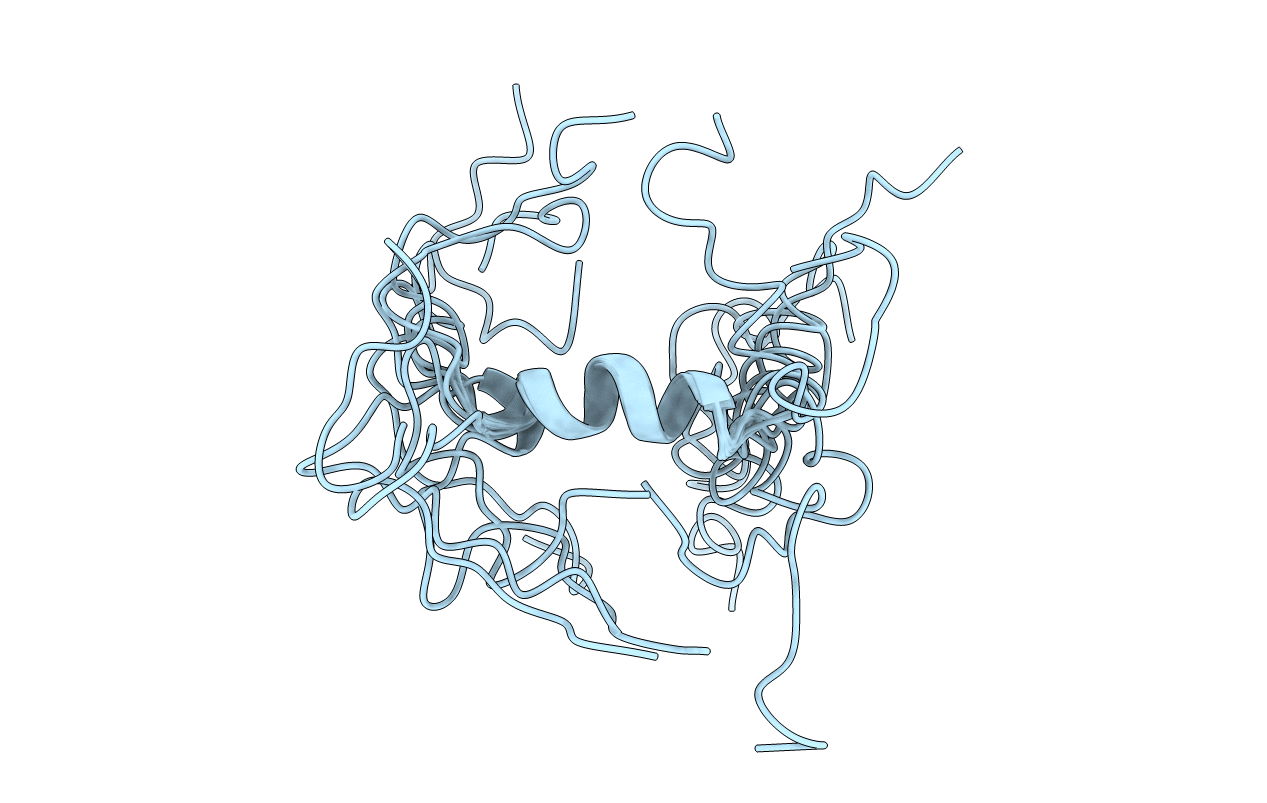
Deposition Date
1998-04-22
Release Date
1998-06-17
Last Version Date
2022-02-16
Entry Detail
PDB ID:
1BA6
Keywords:
Title:
SOLUTION STRUCTURE OF THE METHIONINE-OXIDIZED AMYLOID BETA-PEPTIDE (1-40). DOES OXIDATION AFFECT CONFORMATIONAL SWITCHING? NMR, 10 STRUCTURES
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
50
Conformers Submitted:
10
Selection Criteria:
LOW ENERGY, LOW VIOLATIONS