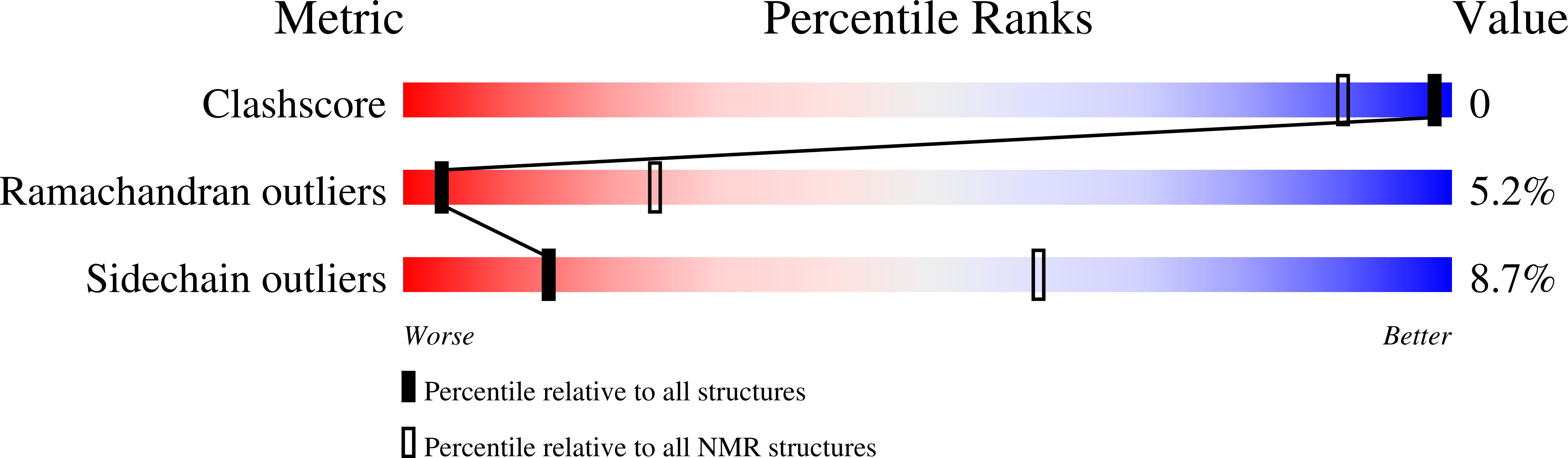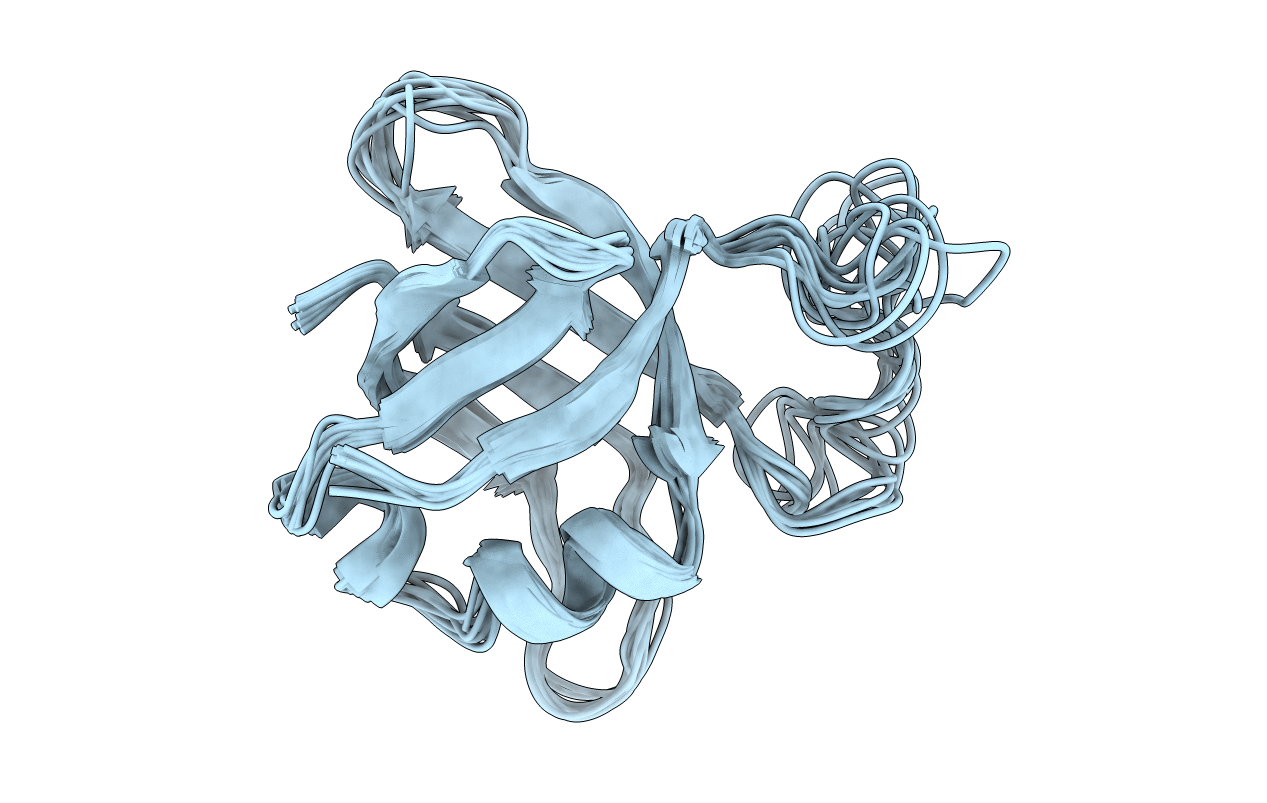
Deposition Date
1999-01-27
Release Date
2000-01-26
Last Version Date
2023-12-27
Entry Detail
PDB ID:
1B75
Keywords:
Title:
SOLUTION STRUCTURE OF RIBOSOMAL PROTEIN L25 FROM ESCHERICHIA COLI
Biological Source:
Source Organism(s):
Escherichia coli (Taxon ID: 562)
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
10
Selection Criteria:
LOWEST TARGET FUNCTION