Deposition Date
1999-01-19
Release Date
1999-01-27
Last Version Date
2024-04-10
Entry Detail
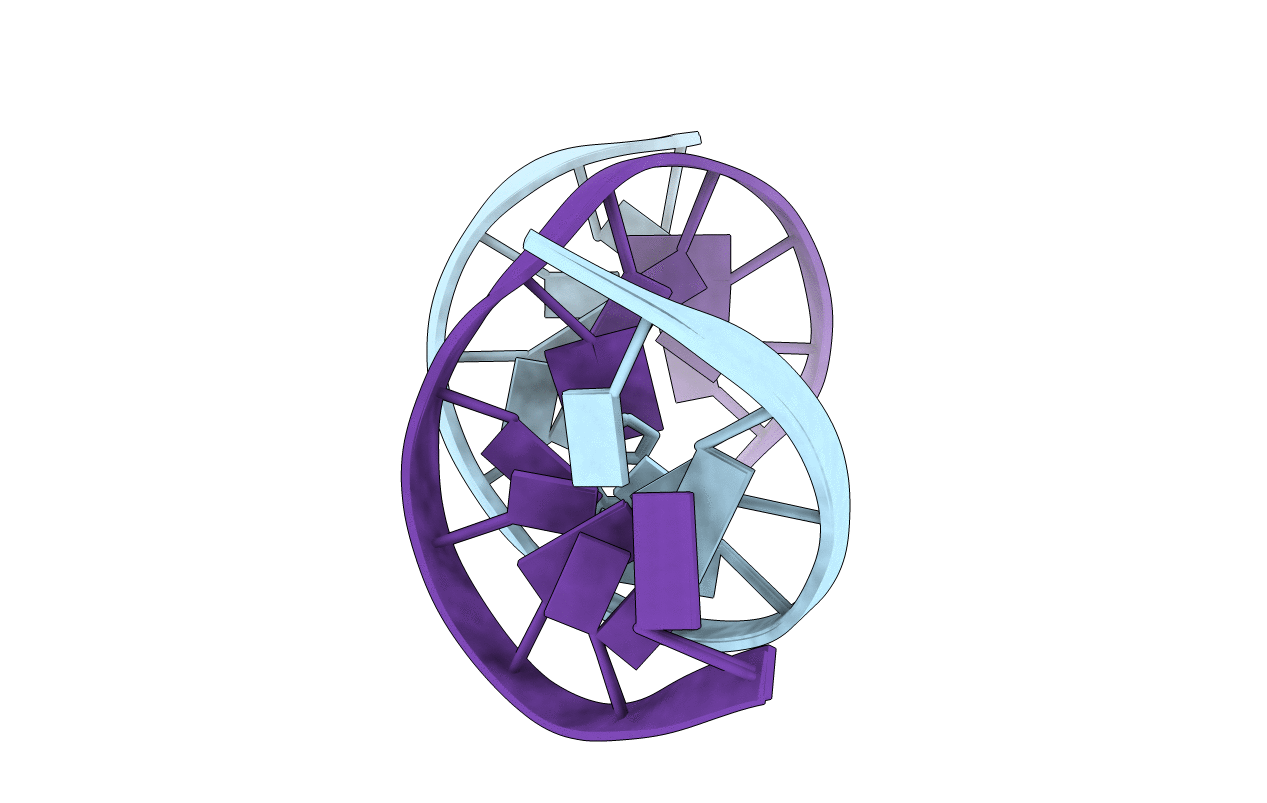
PDB ID:
1B6Y
Keywords:
Title:
3,N4-ETHENO-2'-DEOXYCYTIDINE OPPOSITE ADENINE IN AN 11-MER DUPLEX, SOLUTION STRUCTURE FROM NMR AND MOLECULAR DYNAMICS, 2 STRUCTURES
Method Details:


