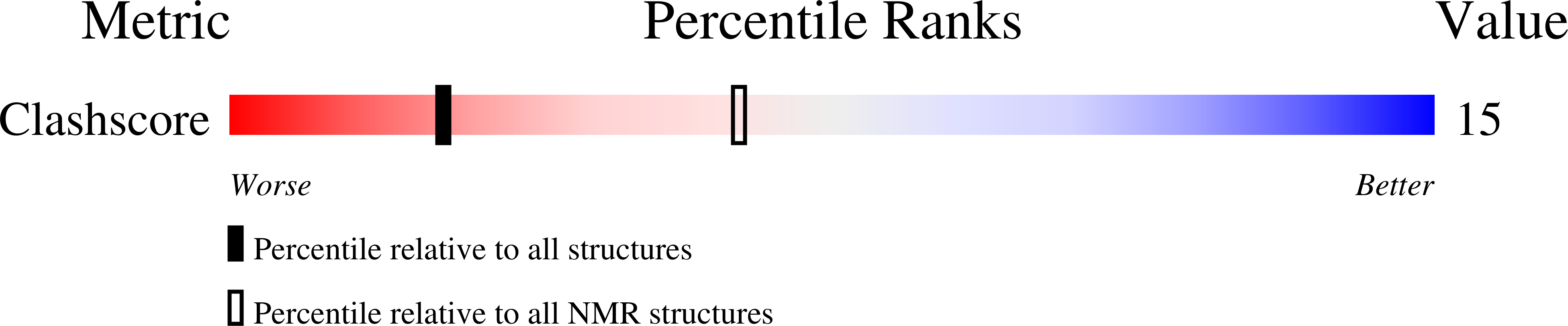
Deposition Date
1997-10-30
Release Date
1998-07-01
Last Version Date
2024-05-22
Entry Detail
PDB ID:
1AX7
Keywords:
Title:
SOLUTION STRUCTURE OF THE [AF]-C8-DG ADDUCT POSITIONED AT A TEMPLATE-PRIMER JUNCTION, NMR, 6 STRUCTURES
Method Details:
Experimental Method:
Conformers Calculated:
6
Conformers Submitted:
6
Selection Criteria:
ALL CONFORMERS PRESENTED