Deposition Date
1997-07-28
Release Date
1998-01-28
Last Version Date
2024-11-20
Entry Detail
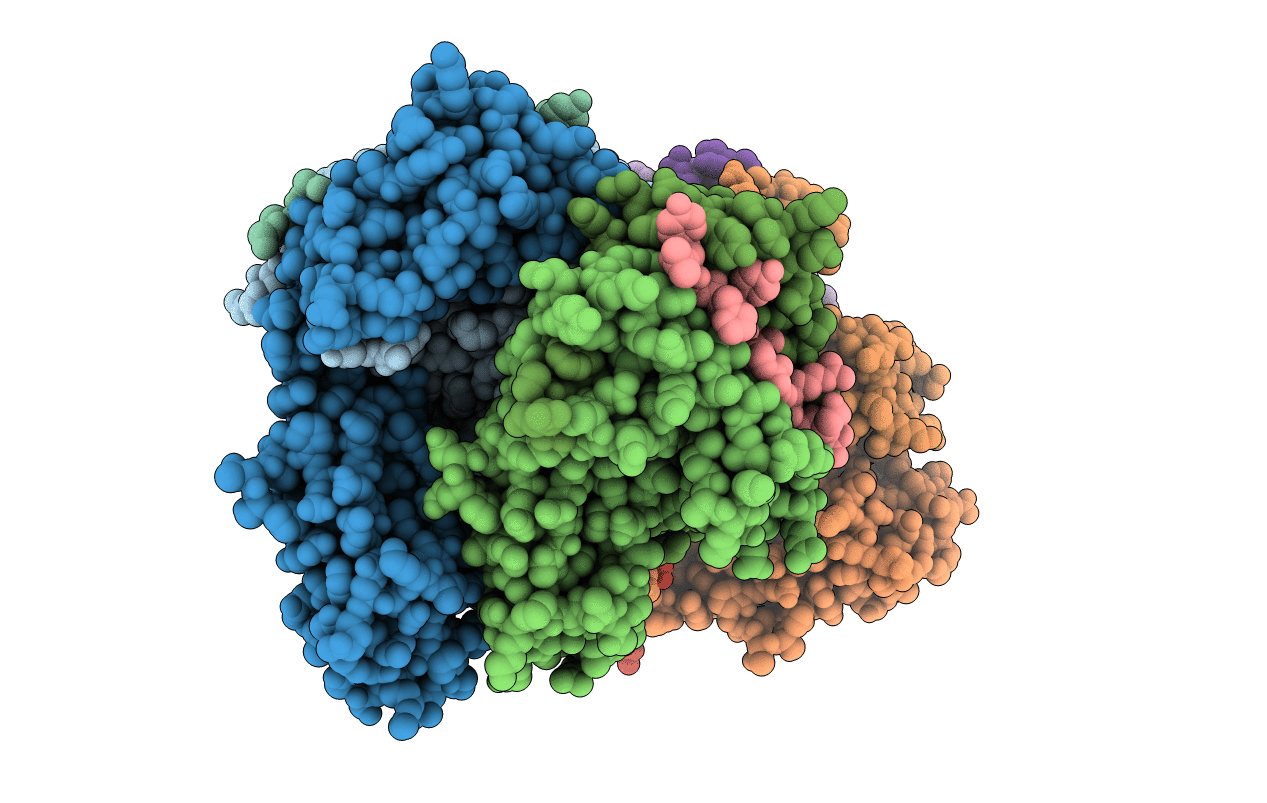
PDB ID:
1AQD
Keywords:
Title:
HLA-DR1 (DRA, DRB1 0101) HUMAN CLASS II HISTOCOMPATIBILITY PROTEIN (EXTRACELLULAR DOMAIN) COMPLEXED WITH ENDOGENOUS PEPTIDE
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.45 Å
R-Value Free:
0.27
R-Value Work:
0.21
R-Value Observed:
0.21
Space Group:
C 1 2 1


