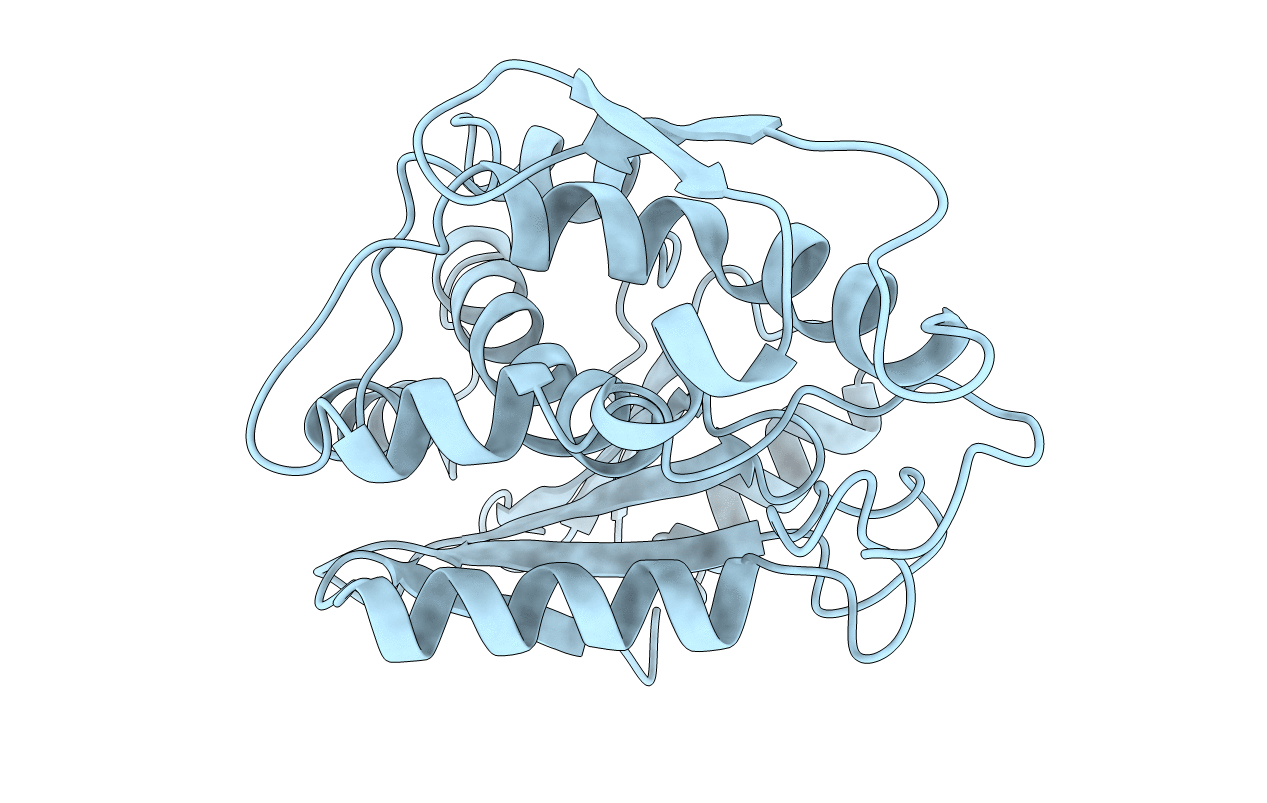
Deposition Date
1993-09-21
Release Date
1994-01-31
Last Version Date
2024-10-16
Entry Detail
PDB ID:
1APA
Keywords:
Title:
X-RAY STRUCTURE OF A POKEWEED ANTIVIRAL PROTEIN, CODED BY A NEW GENOMIC CLONE, AT 0.23 NM RESOLUTION. A MODEL STRUCTURE PROVIDES A SUITABLE ELECTROSTATIC FIELD FOR SUBSTRATE BINDING.
Biological Source:
Source Organism(s):
Phytolacca americana (Taxon ID: 3527)
Method Details:
Experimental Method:
Resolution:
2.30 Å
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
P 21 21 2


