Deposition Date
1997-06-12
Release Date
1997-09-17
Last Version Date
2024-05-22
Entry Detail
PDB ID:
1AL9
Keywords:
Title:
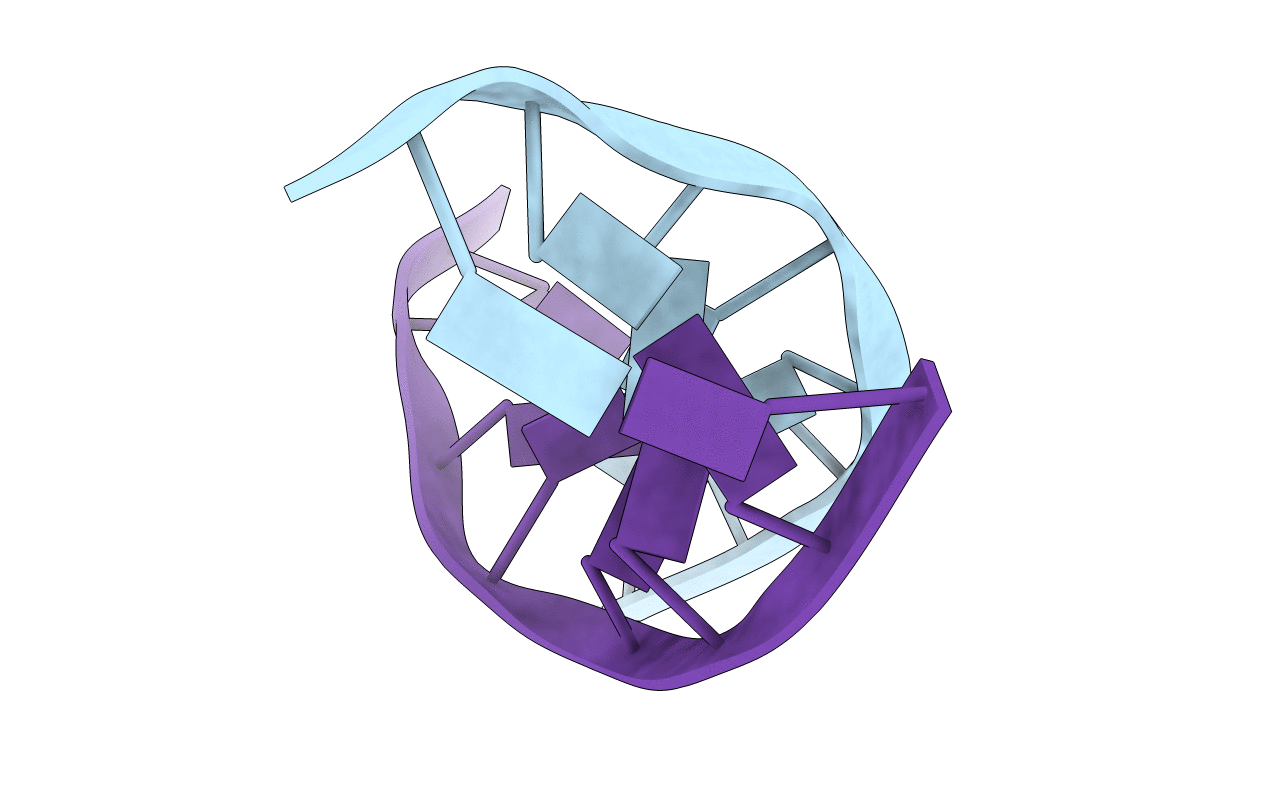
NMR STUDY OF DNA (5'-D(*AP*CP*GP*TP*AP*CP*GP*T)-3') SELF-COMPLEMENTARY DUPLEX COMPLEXED WITH A BIS-DAUNORUBICIN, MINIMIZED AVERAGE STRUCTURE
Method Details:
Experimental Method:
Conformers Calculated:
1
Conformers Submitted:
1
Selection Criteria:
MINIMIZED AVERAGE