Deposition Date
1997-02-10
Release Date
1997-07-07
Last Version Date
2024-10-30
Entry Detail
PDB ID:
1AC0
Keywords:
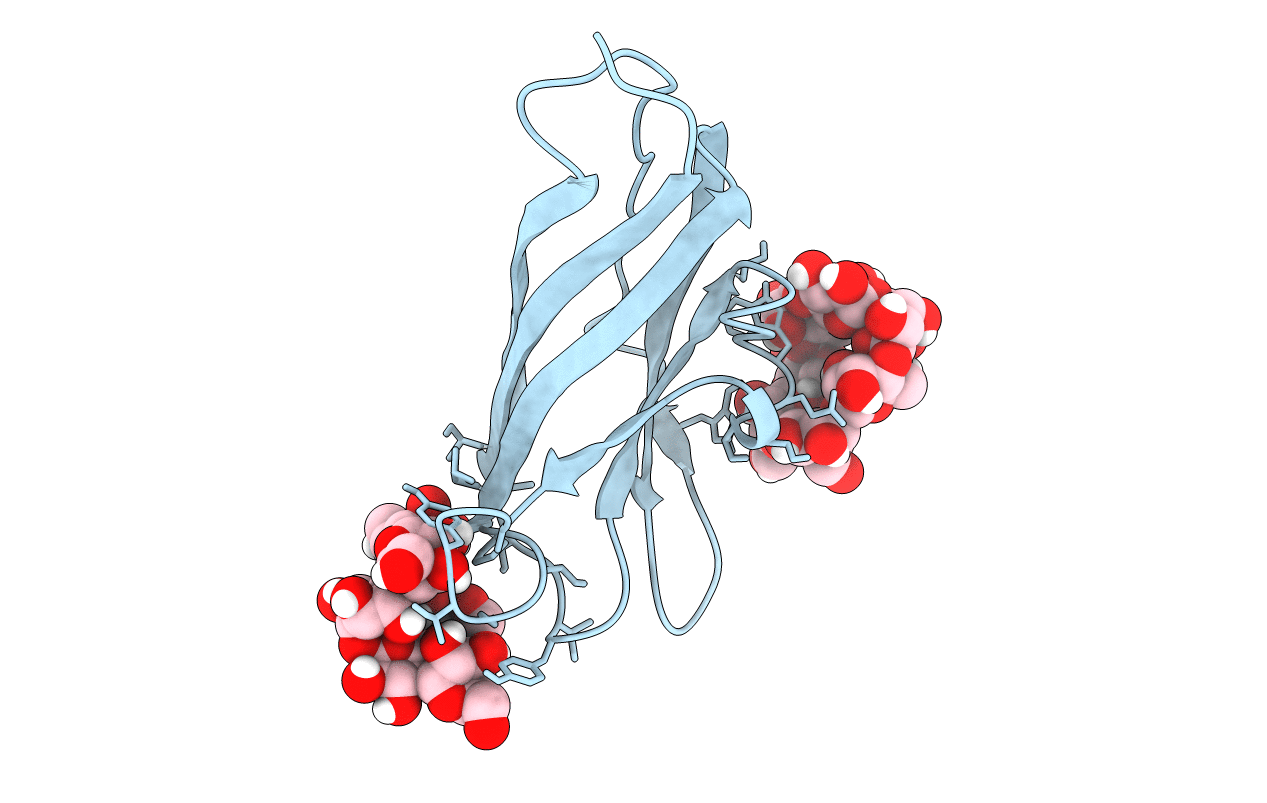
Title:
GLUCOAMYLASE, GRANULAR STARCH-BINDING DOMAIN COMPLEX WITH CYCLODEXTRIN, NMR, MINIMIZED AVERAGE STRUCTURE
Biological Source:
Source Organism(s):
Aspergillus niger (Taxon ID: 5061)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
1
Selection Criteria:
RANDOM FROM 81 GOOD STRUCTURES


