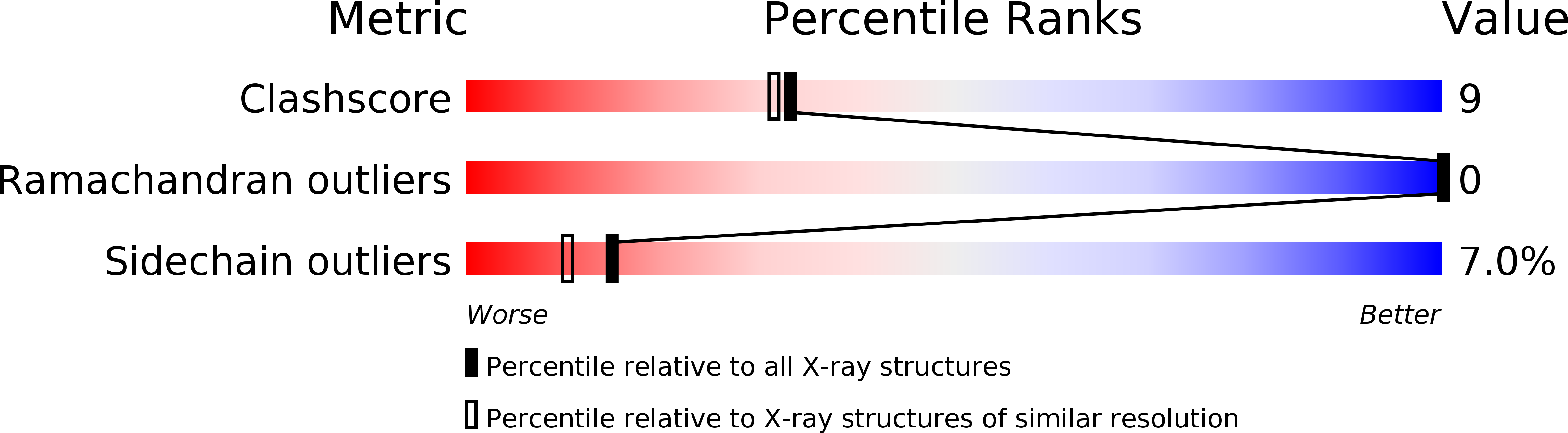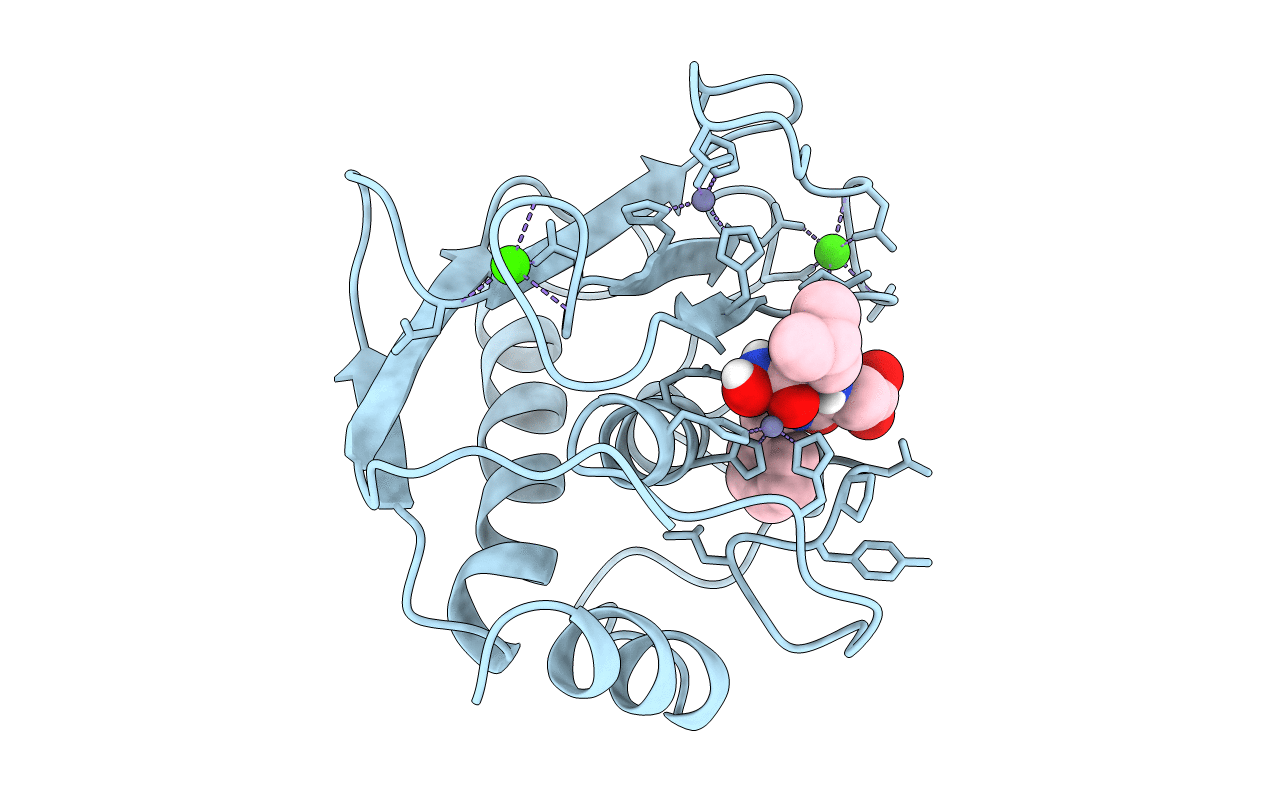
Deposition Date
1998-04-03
Release Date
1999-05-04
Last Version Date
2024-02-07
Entry Detail
PDB ID:
1A86
Keywords:
Title:
MMP8 WITH MALONIC AND ASPARTIC ACID BASED INHIBITOR
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Method Details: